In this issue of Blood, Ji and colleagues assemble evidence to justify transfusion of RhD+ red cells to individuals with RhD− cells in conventional typing but have the common Asian-type DEL mutant.1 Their evidence comes from many sources including direct clinical challenge, epidemiologic studies, and genomic and transcriptomic analysis and has potential implications for millions of people.
RhD is the most important minor (non-ABO) antigen. It is highly antigenic and capable of causing hemolytic transfusion reactions and hemolytic disease of the fetus and newborn.2 In European populations, where the prevalence of RhD− status is approximately 15%, 1 in every 200 children conceived died of Rh disease before anti-RhD immunoglobulin G (IgG) was developed and widely used.3,4 For mothers with RhD−, 1 in 30 of their pregnancies resulted in death from Rh disease.
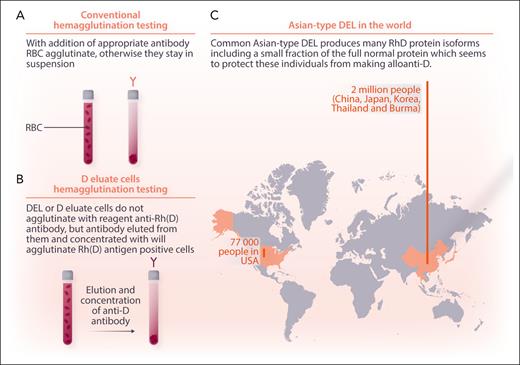
Conventional blood bank testing is performed by hemagglutination, making red cells stick together with antibodies (figure panel A). Hemagglutination testing with strong mouse monoclonal antibody mixes works well for RhD typing in most people, yielding either a strong 4+ reaction or no agglutination. However, individuals with low numbers of RhD protein or aberrant expression of the RhD antigen can test as “weak D.” Pregnant women with weak D are best referred for molecular testing. Some individuals with D− can be shown to have small amounts of RhD antigen present by adding strong anti-D, washing the cells to show the anti-D sticks, and then eluting and concentrating the anti-D and using it to make conventional D+ cells stick together. Such people have the “D eluate” or “DEL” phenotype (figure panel B).
Migration of people with Asian-type DEL to the United States. Conventional blood typing uses combinations of mouse monoclonal antibodies to detect the major Rh protein, “D,” by hemagglutination (A). Donors and patients with the Asian-type DEL mutation make multiple variants of the D protein in reduced amounts, which can be detected by eluting and concentrating the reagent antibodies from their cells and using them to agglutinate reagent RhD+ red blood cells (RBC) (B). Two million such individuals live in China, Japan, Korea, Thailand, and Burma, and tens of thousands live in other countries (C). Professional illustration by Somersault18:24.
Migration of people with Asian-type DEL to the United States. Conventional blood typing uses combinations of mouse monoclonal antibodies to detect the major Rh protein, “D,” by hemagglutination (A). Donors and patients with the Asian-type DEL mutation make multiple variants of the D protein in reduced amounts, which can be detected by eluting and concentrating the reagent antibodies from their cells and using them to agglutinate reagent RhD+ red blood cells (RBC) (B). Two million such individuals live in China, Japan, Korea, Thailand, and Burma, and tens of thousands live in other countries (C). Professional illustration by Somersault18:24.
Of the more than 40 molecular variants that produce the DEL phenotype, the Asian-type, RHD∗1227A (RHD∗01EL.01), is the most common. It occurs in almost 2 million people across China, Japan, Korea, Thailand, Burma, and their descendants around the globe and represents approximately a quarter of everyone who tests as RhD− in these founder countries. Trying to give them all RhD antigen-negative blood strains the blood system resources of these countries, and treating the women of childbearing potential with RhD immunoglobulin is expensive, but no one has proven that doing otherwise is safe. Proving safety, the absence of bad outcomes, is difficult. Just because it has not been seen does not mean it will not occur or that you have not just missed the uncommon bad outcome. To address these concerns, Ji and colleagues used a multipronged approach. First, in a clinical trial and with informed consent, they gave 46 patients with Asian-type DEL RhD+ red cells and showed they did not make anti-D over the subsequent 193+ days. To bolster this direct experiment, they tracked down 4045 women with RhD− who had given birth to children with RhD+ and showed that none of 1037 Asian-type DEL postparturients made an anti-D and that all 79 of the women who made an anti-D were among the 3009 true RhD− subjects. Finally, at a molecular level, they showed that 1 out of 600 transcripts of the RhD gene in these individuals is for the correct protein. This information, in conjunction with previous work showing that monoclonal antibodies against all of the common epitopes of the D protein can be eluted from the DEL variant, suggests that individuals with Asian-type DEL express small amounts of the wild-type protein that protect them from alloimmunization and that RhD antigen-positive RBCs can be administered without causing alloimmunization. We cannot prove safety, but a reasonably prudent individual can now give RhD+ red cells to an individual with Asian-type DEL with outstanding assurance of safety. Ji and colleagues have set a new standard for allelic safety analysis.
Blood from donors with Asian-type DEL blood can cause RhD alloimmunization in individuals with true RhD−.5 There are at least 70 000 individuals with Asian-type DEL living in the United States based on immigrant numbers from the 5 founder countries and more from countries such as the Philippines, Malaysia, Indonesia, and Vietnam with large ethnic Chinese subpopulations (figure panel C). Such donors are not detected by conventional hemagglutination blood donor RhD typing. The utility of screening 2 million RhD− blood donors each year in the United States for the single nucleotide polymorphism of Asian-type DEL compared with other methods of managing residual risk for RhD alloimmunization needs a formal risk assessment, but polymerase chain reaction testing could be piggybacked on current molecular infectious disease testing.6,7 The complexity of ensuring the safety of the blood supply continues to increase.
Conflict of interest: J.R.H. receives author royalties from UpToDate and the AABB, is a consultant to Hemerus, LLC, and is a stockholder in Medcura Inc.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal