Key Points
Subpopulation analyses of Asia enrollees in the POLARIX trial and its Asia extension show consistency with global population results.
PFS hazard ratio was 0.64 for Pola-R-CHP vs R-CHOP and neither peripheral neuropathy nor hepatitis B reactivation were increased.
Abstract
In the phase 3 POLARIX study in previously untreated diffuse large B-cell lymphoma, polatuzumab vedotin combined with rituximab plus cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP) significantly improved progression-free survival (PFS) compared with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with similar safety. Patients were randomized 1:1 to 6 cycles of Pola-R-CHP or R-CHOP plus 2 cycles of rituximab alone. For registration of POLARIX in China, consistency of PFS in an Asia subpopulation (defined as ≥50% of the risk reduction in PFS expected in the global population) was evaluated. Overall, 281 patients were analyzed: 160 patients from Asia in the intention-to-treat (ITT) population of the global study and 121 from an ITT China extension cohort. Of these, 141 were randomized to Pola-R-CHP and 140 to R-CHOP. At data cutoff (28 June 2021; median follow-up 24.2 months), PFS met the consistency definition with the global population, and was superior with Pola-R-CHP vs R-CHOP (hazard ratio, 0.64; 95% confidence interval [CI], 0.40-1.03). Two-year PFS was 74.2% (95% CI, 65.7-82.7) and 66.5% (95% CI, 57.3-75.6) with Pola-R-CHP and R-CHOP, respectively. Safety was comparable between Pola-R-CHP and R-CHOP, including rates of grade 3 to 4 adverse events (AEs; 72.9% vs 66.2%, respectively), serious AEs (32.9% vs 32.4%), grade 5 AEs (1.4% vs 0.7%), AEs leading to study treatment discontinuation (5.0% vs 7.2%), and any-grade peripheral neuropathy (44.3% vs 50.4%). These findings demonstrate consistent efficacy and safety of Pola-R-CHP vs R-CHOP in the Asia and global populations in POLARIX. This trial was registered at https://clinicaltrials.gov/ct2/home as # NCT03274492.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of aggressive non-Hodgkin lymphoma worldwide,1 and in Asia accounts for 40.8%, 36.0%, and 48.4% of all cases in China, Japan, and Korea, respectively.2-4
The current standard of care for first-line (1L) treatment of DLBCL is the anti-CD20 monoclonal antibody rituximab (R) combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).5 Although R-CHOP is an effective therapy in producing clinical response, between 30% and 40% of patients subsequently relapse or become refractory to treatment.5,6 Until recently, attempts to improve upon the efficacy of R-CHOP therapy in DLBCL have not provided meaningful results and R-CHOP remained the standard of care.6
Polatuzumab vedotin comprises an anti-CD79b monoclonal antibody conjugated by a protease-cleavable linker to a potent microtubule inhibitor, monomethyl auristatin E.7;CD79b, a subunit of the B-cell receptor, is ubiquitously expressed on mature B-cell lymphomas including DLBCL;7-9 antibodies bound to CD79b are rapidly internalized, making CD79b ideally suited for targeted delivery of cytotoxic agents.10,11 Polatuzumab vedotin has shown promising efficacy with manageable toxicity in relapsed/refractory DLBCL, both as a single agent12 and in combination with bendamustine and rituximab.13-15 More recently, polatuzumab vedotin in combination with rituximab plus cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP), was investigated in the 1L DLBCL setting in the phase 3 POLARIX study (NCT03274492) and demonstrated superior progression-free survival (PFS) compared with R-CHOP (stratified hazard ratio [HR] 0.73; 95% confidence interval [CI], 0.57-0.95) in patients with previously untreated DLBCL; the safety profiles of the 2 regimens were similar.16
To characterize the efficacy and safety profile of Pola-R-CHP to potentially support a regulatory submission in China, an Asia subpopulation analysis of the POLARIX study was performed. This subpopulation included patients enrolled from Asia during the global study phase, and a China extension cohort, which was enrolled after the global study had completed accrual to increase the number of patients in the study from this country. This manuscript reports the efficacy and safety findings from the Asia subpopulation analysis of POLARIX.
Methods
Trial conduct
Full study details of POLARIX and the protocol have been published previously.16 Patients in the Asia subpopulation, including the China extension cohort, followed the same study design, underwent the same schedule of assessments, and received the same study treatment as the global cohort.
The study protocol was approved by the institutional review boards or ethics committees at participating institutions in accordance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines, including good clinical practice, and the ethical principles originating from the Declaration of Helsinki.17,18 Informed consent was given by all patients.
The POLARIX study was sponsored by Genentech, Inc. and F. Hoffmann-La Roche Ltd, and designed by the sponsor in collaboration with the Lymphoma Study Association. An independent data monitoring committee reviewed data regularly during the conduct of the study.
Patients
Eligible patients were aged between 18 and 80 years with CD20+ DLBCL as defined by the 2016 World Health Organization classification of lymphoid neoplasms,1 had not received previous lymphoma treatment, had an Eastern Cooperative Oncology Group performance status score of 0 to 2, and a baseline International Prognostic Index (IPI)19 score of 2 to 5. Patients with a prior history of hepatitis B virus (HBV) infection (defined as positive total antibody to hepatitis B surface antigen) were permitted if HBV DNA was undetectable by polymerase chain reaction (PCR) at the time of screening.
Exclusion criteria included contraindications to any component of R-CHOP, prior receipt of anthracyclines, and central nervous system involvement.
Randomization, masking, and treatment
Eligible patients were randomized 1:1 to receive Pola-R-CHP or R-CHOP. Randomization was performed centrally and patients, investigators, study site personnel, and the sponsor and their agents were blinded to treatment assignment. In both the global POLARIX population and the China extension cohort, stratification factors included IPI score (IPI 2 vs IPI 3-5) and bulky disease (absent or present, defined as 1 lesion ≥7.5 cm). The global population was also stratified according to geographic region (Western Europe, the United States, Canada, and Australia vs Asia vs rest of world).
All patients were to receive 6, 21-day cycles of treatment. Each cycle consisted of intravenous rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, and doxorubicin 50 mg/m2 on day 1, and oral prednisone 100 mg once daily on days 1 to 5. In addition, either polatuzumab vedotin 1.8 mg/kg and a placebo of vincristine (Pola-R-CHP) or vincristine 1.4 mg/m2 (maximum 2 mg) and a placebo of polatuzumab vedotin (R-CHOP) were administered on day 1. Rituximab 375 mg/m2 was administered as monotherapy in 2 additional cycles. Granulocyte colony-stimulating factor administration was required as primary prophylaxis of neutropenia. For patients with a prior history of HBV infection, monthly monitoring for HBV DNA by PCR was required during treatment and for 12 months after treatment completion. Prophylaxis with antiviral therapy was permitted unless the threshold for HBV DNA testing was >10 IU/mL; reactivation was defined as PCR detection of HBV DNA above this threshold (supplemental Methods, available on the Blood website).
Details regarding dose interruptions, modifications, and discontinuations of polatuzumab vedotin and vincristine, as well as any other permitted treatments (ie, central nervous system prophylaxis, radiotherapy) have been published previously.16
End points and assessments
The primary efficacy end point was investigator-assessed PFS, defined as the time from the date of randomization until the first occurrence of disease progression, relapse, or death from any cause.
Secondary end points included investigator-assessed event-free survival (EFS; defined as the time from date of randomization to the earliest occurrence of disease progression/relapse, death, biopsy that is positive for residual disease after treatment completion, or start of a new antilymphoma therapy because of efficacy reasons), fluorodeoxyglucose positron emission tomography–based complete response (CR) rate at the end of treatment by blinded independent central review, overall survival (OS), and disease-free survival (DFS) in patients achieving a CR. Tumor evaluations were assessed using the Lugano classification of response assessment for lymphoma.19
Antilymphoma treatments received subsequent to study treatment with Pola-R-CHP and R-CHOP were recorded during the trial. All adverse events (AEs) were reported per Medical Dictionary for Regulatory Activities thesaurus terms, with severity graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Central laboratory testing for cell-of-origin classification, protein expression of MYC and BCL2, and rearrangements of MYC, BCL2, and BCL6 were as previously described.16
Statistical analysis
The purpose of the analysis was to evaluate consistency of PFS (as per protocol, defined as ≥50% of the risk reduction in the PFS expected to be observed in the global population) in the Asia subpopulation. Given that the HR for the global population was 0.73, the HR threshold for consistency in this study was 0.87.
The analysis of PFS in the Asia subpopulation was performed when ∼69 PFS events had occurred, which was planned to provide ∼80% probability of observing ≥50% of the risk reduction in the PFS expected to be observed in the global population.
The Asia subpopulation was not powered to demonstrate statistical significance in terms of efficacy, and no formal hypothesis testing was performed. Analysis methods were the same as those used for the global population,16 with the exception that HRs were estimated using unstratified Cox regression models in the Asia subpopulation.
Results
Patients
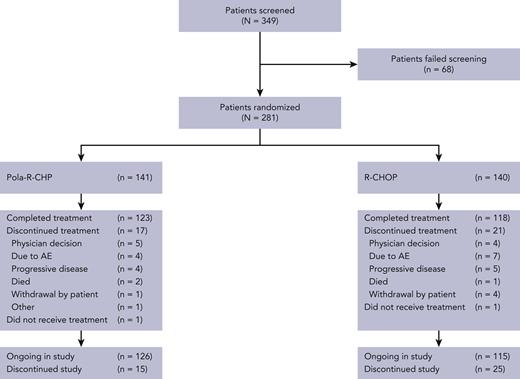
In the global cohort of POLARIX, efficacy analyses were conducted using the intention-to-treat population, of which 160 were from Asia.16 The China extension cohort consisted of 121 patients; the Asia subpopulation therefore consisted of 281 patients in total (mainland China, n = 150; Japan, n = 85; Republic of Korea, n = 31; Taiwan, n = 15; supplemental Figure 1). Of these 281 patients, 141 were randomized to Pola-R-CHP and 140 to R-CHOP. Patient disposition is shown in Figure 1.
Treatment arms were generally balanced for demographic and baseline characteristics (Table 1). For the Pola-R-CHP and R-CHOP arms, the median age was 63 years (range, 19-79 years) and 64 years (range, 23-78), respectively; most patients had an Eastern Cooperative Oncology Group performance status of 0 to 1 (82.3% and 86.4%), an IPI score of 3 to 5 (61.7% and 62.1%), advanced Ann Arbor stage III-IV (86.5% and 85.0%), and elevated lactate dehydrogenase levels (68.1% and 70.7%). There were no imbalances in stratification factors, IPI score, or bulky disease between arms. One observed difference was the median time from diagnosis to initiation of treatment, which was 17 days in the Pola-R-CHP arm and 15 days in the R-CHOP arm (compared with 26-27 days in the global study). Overall, the demographics and baseline disease characteristics of the Asia subpopulation were generally similar to those of the global population16 (supplemental Table 1). Exceptions were that the Asia subpopulation were younger, fewer patients had baseline bulky disease, and a slightly higher proportion of patients had activated B-cell (ABC) cell of origin than in the global population.
Baseline patient demographics and clinical characteristics (intention-to-treat population)
| . | Pola-R-CHP (n = 141) . | R-CHOP (n = 140) . |
|---|---|---|
| Median age (range), y | 63 (19-79) | 64 (23-78) |
| Age, n (%) | ||
| <65 y | 83 (58.9) | 73 (52.1) |
| ≥65 y | 58 (41.1) | 67 (47.9) |
| Sex, n (%) | ||
| Female | 70 (49.6) | 62 (44.3) |
| Male | 71 (50.4) | 78 (55.7) |
| Country, n (%) | ||
| Mainland China | 75 (53.2) | 75 (53.6) |
| Japan | 43 (30.5) | 42 (30.0) |
| Republic of Korea | 15 (10.6) | 16 (11.4) |
| Taiwan | 8 (5.7) | 7 (5.0) |
| Ann Arbor stage, n (%) | ||
| I-II | 19 (13.5) | 21 (15.0) |
| III-IV | 122 (86.5) | 119 (85.0) |
| Extranodal sites, n (%) | ||
| 0-1 | 77 (54.6) | 82 (58.6) |
| ≥2 | 64 (45.4) | 58 (41.4) |
| Bulky disease, n (%)∗ | ||
| <7.5 cm | 97 (68.8) | 93 (66.4) |
| ≥7.5 cm | 44 (31.2) | 47 (33.6) |
| ECOG PS, n (%) | ||
| 0-1 | 116 (82.3) | 121 (86.4) |
| 2 | 25 (17.7) | 19 (13.6) |
| LDH level, n (%) | ||
| Normal | 45 (31.9) | 41 (29.3) |
| Elevated | 96 (68.1) | 99 (70.7) |
| IPI score, n (%)∗ | ||
| 2 | 54 (38.3) | 53 (37.9) |
| 3–5 | 87 (61.7) | 87 (62.1) |
| Median time from initial diagnosis to treatment initiation (IQR), days | 17 (10-29) | 15 (10-26) |
| Cell of origin, n (%)† | n = 100 | n = 100 |
| GCB | 30 (30.0) | 40 (40.0) |
| ABC | 55 (55.0) | 46 (46.0) |
| Unclassified | 15 (15.0) | 14 (14.0) |
| DEL, n (%)† | n = 110 | n = 113 |
| DEL | 35 (31.8) | 41 (36.3) |
| Non-DEL | 75 (68.2) | 72 (63.7) |
| Double-/triple-hit lymphoma, n (%)† | n = 103 | n = 99 |
| Yes | 3 (2.9) | 5 (5.1) |
| No | 100 (97.1) | 94 (94.9) |
| Previous history of hepatitis B infection‡ | 45 (32.1)§ | 59 (42.4)§ |
| . | Pola-R-CHP (n = 141) . | R-CHOP (n = 140) . |
|---|---|---|
| Median age (range), y | 63 (19-79) | 64 (23-78) |
| Age, n (%) | ||
| <65 y | 83 (58.9) | 73 (52.1) |
| ≥65 y | 58 (41.1) | 67 (47.9) |
| Sex, n (%) | ||
| Female | 70 (49.6) | 62 (44.3) |
| Male | 71 (50.4) | 78 (55.7) |
| Country, n (%) | ||
| Mainland China | 75 (53.2) | 75 (53.6) |
| Japan | 43 (30.5) | 42 (30.0) |
| Republic of Korea | 15 (10.6) | 16 (11.4) |
| Taiwan | 8 (5.7) | 7 (5.0) |
| Ann Arbor stage, n (%) | ||
| I-II | 19 (13.5) | 21 (15.0) |
| III-IV | 122 (86.5) | 119 (85.0) |
| Extranodal sites, n (%) | ||
| 0-1 | 77 (54.6) | 82 (58.6) |
| ≥2 | 64 (45.4) | 58 (41.4) |
| Bulky disease, n (%)∗ | ||
| <7.5 cm | 97 (68.8) | 93 (66.4) |
| ≥7.5 cm | 44 (31.2) | 47 (33.6) |
| ECOG PS, n (%) | ||
| 0-1 | 116 (82.3) | 121 (86.4) |
| 2 | 25 (17.7) | 19 (13.6) |
| LDH level, n (%) | ||
| Normal | 45 (31.9) | 41 (29.3) |
| Elevated | 96 (68.1) | 99 (70.7) |
| IPI score, n (%)∗ | ||
| 2 | 54 (38.3) | 53 (37.9) |
| 3–5 | 87 (61.7) | 87 (62.1) |
| Median time from initial diagnosis to treatment initiation (IQR), days | 17 (10-29) | 15 (10-26) |
| Cell of origin, n (%)† | n = 100 | n = 100 |
| GCB | 30 (30.0) | 40 (40.0) |
| ABC | 55 (55.0) | 46 (46.0) |
| Unclassified | 15 (15.0) | 14 (14.0) |
| DEL, n (%)† | n = 110 | n = 113 |
| DEL | 35 (31.8) | 41 (36.3) |
| Non-DEL | 75 (68.2) | 72 (63.7) |
| Double-/triple-hit lymphoma, n (%)† | n = 103 | n = 99 |
| Yes | 3 (2.9) | 5 (5.1) |
| No | 100 (97.1) | 94 (94.9) |
| Previous history of hepatitis B infection‡ | 45 (32.1)§ | 59 (42.4)§ |
DEL, double-expressor lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal center B-cell subtype; IQR, interquartile range; LDH, lactate dehydrogenase.
Based on stratification.
Centrally performed tests: cell-of-origin identification was performed by NanoString Lymph 2Cx; MYC and BCL2 immunohistochemistry were performed for DEL; MYC and BCL2, and/or BCL6 rearrangements were performed for double- and triple-hit lymphoma; percentages are calculated from the evaluable population. The remainder of the results were considered unknown and/or represent test failures.
Based on laboratory data at screening (hepatitis B core antibody detected).
Based on the safety-evaluable population; Pola-R-CHP, n = 140; R-CHOP, n = 139.
Treatment exposure
A total of 279 patients received ≥1 dose of study treatment (140 patients in the Pola-R-CHP arm and 139 patients in the R-CHOP arm) and were included in the safety-evaluable population, and most patients received all planned doses of chemotherapy. Few patients in either arm discontinued study treatment early, with 94.3% and 89.2% of patients in the Pola-R-CHP and R-CHOP arms, respectively, receiving ≥6 cycles of any study drug; 87.9% and 84.9% of patients, respectively, received 8 cycles of rituximab.
Most patients received all 6 doses of the active blinded agents, polatuzumab vedotin or vincristine; 92.9% of patients received all 6 planned doses of polatuzumab vedotin in the Pola-R-CHP arm and 87.1% of patients received all 6 planned doses of vincristine in the R-CHOP arm.
The median relative dose intensities of rituximab, doxorubicin, and cyclophosphamide were 100% in both treatment arms.
Efficacy
Primary end point
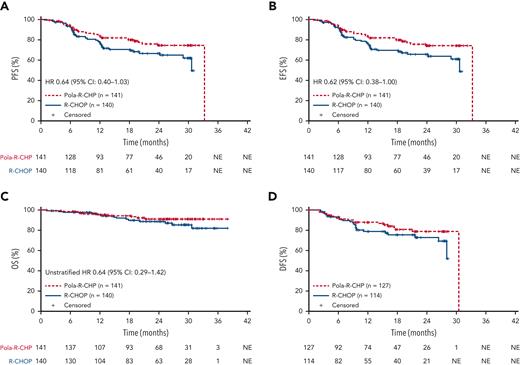
At data cutoff, 28 June 2021 (median follow-up of 24.2 months), the HR for PFS was 0.64; 95% CI, 0.40 to 1.03 (Table 2; Figure 2A), and met the preplanned definition for PFS consistency with the global study population. The proportion of patients remaining progression free at 2 years was 74.2% (95% CI, 65.7-82.7) in the Pola-R-CHP arm and 66.5% (95% CI, 57.3-75.6) in the R-CHOP arm (Table 2).
Primary and key secondary efficacy outcomes (intention-to-treat population)
| Outcome . | Pola-R-CHP (n = 141) . | R-CHOP (n = 140) . |
|---|---|---|
| PFS, number of events (%)∗ | 30 (21.3) | 40 (28.6) |
| Death, n | 2 | 4 |
| Progression or relapse, n | 28 | 36 |
| HR (95% CI) | 0.64 (0.40-1.03) | |
| 2-y rate (95% CI) | 74.2 (65.7-82.7) | 66.5 (57.3-75.6) |
| EFS, number of events (%)∗ | 30 (21.3) | 41 (29.3) |
| Death, n | 2 | 4 |
| Progression or relapse, n | 24 | 33 |
| Other†, n | 4 | 4 |
| HR (95% CI) | 0.62 (0.38-1.00) | |
| 2-y rate (95% CI) | 74.2 (65.7-82.7) | 65.6 (56.4-74.8) |
| ORR at EOT, n (%)‡ | 127 (90.1) | 114 (81.4) |
| CR, n (%) | 116 (82.3) | 109 (77.9) |
| PR, n (%) | 11 (7.8) | 5 (3.6) |
| SD, n (%) | 3 (2.1) | 5 (3.6) |
| PD, n (%) | 4 (2.8) | 10 (7.1) |
| Nonevaluable | 1 (0.7) | 3 (2.1) |
| Not included/missing | 6 (4.3) | 8 (5.7) |
| OS events, n (%) | 10 (7.1) | 15 (10.7) |
| HR (95% CI) | 0.64 (0.29-1.42) | |
| 2-y rate (95% CI) | 91.1 (85.7-96.5) | 88.7 (82.6-94.8) |
| DFS events, n (%)§ | 20 (15.7) | 24 (21.1) |
| Unstratified HR (95% CI) | 0.67 (0.37-1.23) |
| Outcome . | Pola-R-CHP (n = 141) . | R-CHOP (n = 140) . |
|---|---|---|
| PFS, number of events (%)∗ | 30 (21.3) | 40 (28.6) |
| Death, n | 2 | 4 |
| Progression or relapse, n | 28 | 36 |
| HR (95% CI) | 0.64 (0.40-1.03) | |
| 2-y rate (95% CI) | 74.2 (65.7-82.7) | 66.5 (57.3-75.6) |
| EFS, number of events (%)∗ | 30 (21.3) | 41 (29.3) |
| Death, n | 2 | 4 |
| Progression or relapse, n | 24 | 33 |
| Other†, n | 4 | 4 |
| HR (95% CI) | 0.62 (0.38-1.00) | |
| 2-y rate (95% CI) | 74.2 (65.7-82.7) | 65.6 (56.4-74.8) |
| ORR at EOT, n (%)‡ | 127 (90.1) | 114 (81.4) |
| CR, n (%) | 116 (82.3) | 109 (77.9) |
| PR, n (%) | 11 (7.8) | 5 (3.6) |
| SD, n (%) | 3 (2.1) | 5 (3.6) |
| PD, n (%) | 4 (2.8) | 10 (7.1) |
| Nonevaluable | 1 (0.7) | 3 (2.1) |
| Not included/missing | 6 (4.3) | 8 (5.7) |
| OS events, n (%) | 10 (7.1) | 15 (10.7) |
| HR (95% CI) | 0.64 (0.29-1.42) | |
| 2-y rate (95% CI) | 91.1 (85.7-96.5) | 88.7 (82.6-94.8) |
| DFS events, n (%)§ | 20 (15.7) | 24 (21.1) |
| Unstratified HR (95% CI) | 0.67 (0.37-1.23) |
BICR, blinded independent central review; EOT, end of treatment; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
As assessed by investigator.
New antilymphoma therapy or positive biopsy.
As assessed by BICR.
Pola-R-CHP, n = 127; R-CHOP, n = 114. Patients with an investigator-assessed best response of CR at any time during the study were evaluable for DFS.
Kaplan–Meier estimates of efficacy end points. (A) Investigator-assessed PFS. (B) Investigator-assessed EFS. (C) OS. (D) DFS (intention-to-treat population). Data cutoff: 28 June, 2021. EFS, EFS for efficacy purposes.
Kaplan–Meier estimates of efficacy end points. (A) Investigator-assessed PFS. (B) Investigator-assessed EFS. (C) OS. (D) DFS (intention-to-treat population). Data cutoff: 28 June, 2021. EFS, EFS for efficacy purposes.
Secondary end points
Key secondary efficacy results for the Asia subpopulation were supportive of the primary end point. EFS outcomes were consistent with results of the primary end point PFS (unstratified HR, 0.62; 95% CI, 0.38-1.00); 2-year EFS was 74.2% (95% CI, 65.7-82.7) with Pola-R-CHP and 65.6% (95% CI, 56.4-74.8) with R-CHOP (Table 2; Figure 2B). Blinded independent central review-assessed CR rates at end of treatment by fluorodeoxyglucose positron emission tomography were 82.3% (95% CI, 75.0-88.2) with Pola-R-CHP and 77.9% (95% CI, 70.1-84.4) with R-CHOP. OS data were immature at the time of analysis (Pola-R-CHP: 10 deaths [7.1%]; R-CHOP: 15 deaths [10.7%]; unstratified HR, 0.64; 95% CI, 0.29-1.42; Table 2; Figure 2C). In patients who achieved CR, the HR for DFS was 0.67 (unstratified; 95% CI 0.37-1.23; Table 2; Figure 2D). Primary and key secondary efficacy outcomes for the China extension cohort are presented in supplemental Table 2.
Subsequent antilymphoma therapy
Similar to the PFS findings, fewer patients experienced disease relapse and received subsequent therapy in the Pola-R-CHP arm than in the R-CHOP arm. At time of data cutoff, 22.0% and 32.9% of patients in the Pola-R-CHP and R-CHOP arms, respectively, received ≥1 subsequent antilymphoma therapies (supplemental Table 3).
Safety
An overview of key safety data from this Asia subpopulation analysis are presented in Tables 3 and 4. Safety data for the China extension cohort are shown in supplemental Table 4.
Treatment-emergent AEs (safety-evaluable population)
| AEs, n (%) . | Pola-R-CHP (n = 140) . | R-CHOP (n = 139) . |
|---|---|---|
| Any grade | 139 (99.3) | 138 (99.3) |
| Grade ≥3 | 104 (74.3) | 93 (66.9) |
| Serious | 46 (32.9) | 45 (32.4) |
| Grade 5 | 2 (1.4) | 1 (0.7) |
| Most common any-grade AEs∗, n (%) | ||
| Anemia | 61 (43.6) | 55 (39.6) |
| Alopecia | 56 (40.0) | 51 (36.7) |
| Neutrophil count decreased† | 50 (35.7) | 48 (34.5) |
| White blood cell count decreased† | 52 (37.1) | 43 (30.9) |
| Peripheral sensory neuropathy | 41 (29.3) | 49 (35.3) |
| Neutropenia | 47 (33.6) | 40 (28.8) |
| Nausea | 44 (31.4) | 38 (27.3) |
| Constipation | 33 (23.6) | 39 (28.1) |
| Platelet count decreased | 32 (22.9) | 31 (22.3) |
| Leukopenia | 29 (20.7) | 31 (22.3) |
| ALT increased | 27 (19.3) | 26 (18.7) |
| Pyrexia | 25 (17.9) | 27 (19.4) |
| Decreased appetite | 28 (20.0) | 23 (16.5) |
| Lymphocyte count decreased† | 25 (17.9) | 26 (18.7) |
| Weight decreased | 21 (15.0) | 24 (17.3) |
| Diarrhea | 25 (17.9) | 18 (12.9) |
| Pneumonia | 22 (15.7) | 21 (15.1) |
| AST increased | 21 (15.0) | 21 (15.1) |
| Thrombocytopenia | 23 (16.4) | 17 (12.2) |
| Hypokalemia | 13 (9.3) | 21 (15.1) |
| AEs, n (%) . | Pola-R-CHP (n = 140) . | R-CHOP (n = 139) . |
|---|---|---|
| Any grade | 139 (99.3) | 138 (99.3) |
| Grade ≥3 | 104 (74.3) | 93 (66.9) |
| Serious | 46 (32.9) | 45 (32.4) |
| Grade 5 | 2 (1.4) | 1 (0.7) |
| Most common any-grade AEs∗, n (%) | ||
| Anemia | 61 (43.6) | 55 (39.6) |
| Alopecia | 56 (40.0) | 51 (36.7) |
| Neutrophil count decreased† | 50 (35.7) | 48 (34.5) |
| White blood cell count decreased† | 52 (37.1) | 43 (30.9) |
| Peripheral sensory neuropathy | 41 (29.3) | 49 (35.3) |
| Neutropenia | 47 (33.6) | 40 (28.8) |
| Nausea | 44 (31.4) | 38 (27.3) |
| Constipation | 33 (23.6) | 39 (28.1) |
| Platelet count decreased | 32 (22.9) | 31 (22.3) |
| Leukopenia | 29 (20.7) | 31 (22.3) |
| ALT increased | 27 (19.3) | 26 (18.7) |
| Pyrexia | 25 (17.9) | 27 (19.4) |
| Decreased appetite | 28 (20.0) | 23 (16.5) |
| Lymphocyte count decreased† | 25 (17.9) | 26 (18.7) |
| Weight decreased | 21 (15.0) | 24 (17.3) |
| Diarrhea | 25 (17.9) | 18 (12.9) |
| Pneumonia | 22 (15.7) | 21 (15.1) |
| AST increased | 21 (15.0) | 21 (15.1) |
| Thrombocytopenia | 23 (16.4) | 17 (12.2) |
| Hypokalemia | 13 (9.3) | 21 (15.1) |
AEs are MedDRA version 24.0 preferred terms.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; MedDRA, Medical Dictionary for Regulatory Activities.
Most common was defined as all-grade AEs occurring in ≥15% of patients in any treatment arm.
Finding based on laboratory test results indicating a decrease in the number of neutrophils, white blood cells, or lymphocyte count in a blood specimen. Please note that for the AE, neutrophil count decreased, the term “neutropenia” may also have been listed by investigators.
Treatment-emergent grade 3 to 4 AEs (safety-evaluable population)
| Most common AEs,∗ n (%) . | Pola-R-CHP (n = 140) . | R-CHOP (n = 139) . |
|---|---|---|
| Neutropenia | 42 (30.0) | 37 (26.6) |
| Neutrophil count decreased† | 42 (30.0) | 34 (24.5) |
| White blood cell count decreased† | 38 (27.1) | 29 (20.9) |
| Leukopenia | 23 (16.4) | 23 (16.5) |
| Febrile neutropenia | 17 (12.1) | 14 (10.1) |
| Anemia | 12 (8.6) | 17 (12.2) |
| Lymphocyte count decreased† | 15 (10.7) | 12 (8.6) |
| Platelet count decreased | 9 (6.4) | 11 (7.9) |
| Pneumonia | 9 (6.4) | 10 (7.2) |
| Most common AEs,∗ n (%) . | Pola-R-CHP (n = 140) . | R-CHOP (n = 139) . |
|---|---|---|
| Neutropenia | 42 (30.0) | 37 (26.6) |
| Neutrophil count decreased† | 42 (30.0) | 34 (24.5) |
| White blood cell count decreased† | 38 (27.1) | 29 (20.9) |
| Leukopenia | 23 (16.4) | 23 (16.5) |
| Febrile neutropenia | 17 (12.1) | 14 (10.1) |
| Anemia | 12 (8.6) | 17 (12.2) |
| Lymphocyte count decreased† | 15 (10.7) | 12 (8.6) |
| Platelet count decreased | 9 (6.4) | 11 (7.9) |
| Pneumonia | 9 (6.4) | 10 (7.2) |
AEs are MedDRA version 24.0 preferred terms.
MedDRA, the Medical Dictionary for Regulatory Activities.
Most common was defined as grade 3 to 4 AEs occurring in ≥5% of patients in any treatment arm.
Finding based on laboratory test results indicating a decrease in the number of white blood cells in a blood specimen.
The incidence of AEs of any grade was 99.3% in both arms. Table 3 provides a summary of any-grade treatment-emergent AEs; the most common AEs were anemia (Pola-R-CHP, 43.6% and R-CHOP, 39.6%), alopecia (40.0% and 36.7%), and neutrophil count decreased (35.7% and 34.5%). Rates of grade 3 to 4 AEs (72.9% and 66.2%) and serious AEs (32.9% and 32.4%) were comparable between treatment arms. The most common treatment-emergent grade 3 to 4 AEs by preferred term were neutropenia (30.0% and 26.6%), neutrophil count decreased (30.0% and 24.5%), and white blood cell count decreased (27.1% and 20.9%; Table 4). There were 2 (1.4%) grade 5 AEs in the Pola-R-CHP arm (suspected COVID-19 and unexplained death), and 1 (0.7%) grade 5 AE in the R-CHOP arm (unexplained death).
The overall incidence of AEs leading to any study treatment discontinuation was 5.0% in the Pola-R-CHP arm and 7.2% in the R-CHOP arm. Four patients (2.9%) in the Pola-R-CHP arm discontinued polatuzumab vedotin because of AEs and 7 patients (5.0%) in the R-CHOP arm discontinued vincristine because of AEs. The proportion of patients who experienced ≥1 AE leading to any study treatment dose reduction was 8.6% with Pola-R-CHP and 11.5% with R-CHOP.
The incidence of neutropenia as a group term (which included the following preferred terms from the system organ class of neutropenia: neutropenia, neutrophil count decrease, white blood cell decrease, and febrile neutropenia) with Pola-R-CHP and R-CHOP was 67.9% and 60.4%, respectively, for any grade, and 30.0% and 26.6%, respectively, for grade 3 to 4. The incidence of febrile neutropenia as a preferred term was 12.1% with Pola-R-CHP and 10.8% with R-CHOP. The proportion of patients experiencing drug discontinuations (0.7% and 0.0%) or dose reductions (3.6% and 2.9%) because of neutropenia as a group term was comparable with Pola-R-CHP and R-CHOP, as were any-grade infections (41.4% and 48.2%), grade 3 to 4 infections (10.0% and 15.8%), or drug discontinuations (2.9% and 3.6%) and dose reductions (0.7% and 1.4%) because of infections.
The incidence of peripheral neuropathy (PN) was 44.3% with Pola-R-CHP and 50.4% with R-CHOP; the severity of PN was similar between arms. All events were grade 1 to 2 (5.7% and 5.8% of patients experienced grade 2 PN in the Pola-R-CHP and R-CHOP arms, respectively), with the exception of 1 patient with a grade 3 event in the R-CHOP arm. Among patients who developed neuropathy, the median time to onset of any neuropathy was 2.2 months with Pola-R-CHP and 0.8 months with R-CHOP, and the median time to resolution of any neuropathy was 8.1 and 5.6 months, respectively. Very few patients discontinued any study treatment because of PN (none with Pola-R-CHP, and 2 patients [1.4%] with R-CHOP), whereas few patients had reduced polatuzumab vedotin or vincristine dosing because of PN (1.4% [n = 2] and 2.9% [n = 4], respectively).
At screening, based on laboratory data (hepatitis B core antibody detected), 45 (32.1%) patients in the Pola-R-CHP arm and 59 (42.4%) patients in the R-CHOP arm had a previous history of HBV infection. HBV reactivation was defined as PCR DNA detection any time after baseline analysis. Of these patients that were at risk of HBV reactivation, HBV prophylaxis was administered in 21 (46.7%) patients in the Pola-R-CHP arm and 30 (50.8%) in the R-CHOP arm. Two (4.4%) patients in the Pola-R-CHP arm and 11 (18.6%) in the R-CHOP arm experienced HBV reactivation any time after baseline analysis (supplemental Table 5); among patients who received HBV antiviral prophylaxis, 1 (4.8%) and 8 (26.7%) in the Pola-R-CHP arm and R-CHOP arms, respectively, experienced HBV reactivation. Five patients in the R-CHOP arm experienced HBV reactivation during treatment and the remaining 8 patients (2 in the Pola-R-CHP arm and 6 in the R-CHOP arm) experienced reactivation during follow-up. No cases of fulminant hepatitis B occurred.
Discussion
The results of the Asia subpopulation PFS analysis (HR 0.64) met the criteria for consistency with the primary findings of the POLARIX global study population.16 Secondary efficacy analyses were supportive of the primary end point findings, showing benefit of Pola-R-CHP with regard to EFS, CR rate at treatment completion, and DFS. OS data were immature at the time of the analysis but there were fewer deaths in the Pola-R-CHP arm than the R-CHOP arm.
Pola-R-CHP in patients with previously untreated DLBCL was generally well tolerated in the Asia subpopulation, and toxicities were manageable. As observed in the global study cohort,16 the safety profile of the Pola-R-CHP regimen was comparable to R-CHOP and in line with the known safety profiles of each individual component and the underlying disease. The incidence of neutropenia, febrile neutropenia, and infections were generally similar between treatment arms, as were the overall rates of grade 3 to 4 AEs and serious AEs. A comparable incidence of AEs leading to any treatment discontinuations or dose reductions in the Pola-R-CHP and R-CHOP arms was noted, suggesting that Pola-R-CHP has good tolerability and that replacement of vincristine with polatuzumab vedotin did not have a negative impact on the therapeutic course in the Asia subpopulation studied. The incidence and severity of PN was similar between treatment arms, and the proportion of patients experiencing this AE with Pola-R-CHP was lower in the Asia subpopulation (43.4%) than reported for the global study population (52.9%).16
With regards to HBV (which is highly endemic across most of Asia and moderately endemic in Japan20), almost a third of patients in the Pola-R-CHP arm of the Asia subpopulation had evidence of prior infection at screening and were considered at risk of HBV reactivation. However, the rate of reactivation was relatively low in this arm (<5%), despite the known risks associated with rituximab,20 steroid,21,22 and anthracycline23 usage. Of patients with prior HBV infection enrolled in this study, 4.4% in the Pola-R-CHP arm and 18.6% in the R-CHOP arm had evidence of reactivation any time after baseline analysis. Because the population with prior HBV infection represented a small number of patients, no firm conclusions can be drawn; however, there did not appear to be a greater incidence of HBV reactivation with Pola-R-CHP than with R-CHOP. In addition, this finding was not attributable to differences in the proportion of patients receiving routine HBV antiviral prophylaxis vs HBV DNA PCR monitoring to guide therapy. These findings suggest that Pola-R-CHP is well tolerated with regards to HBV reactivation in patients that are at-risk; however, the need for continued vigilance in this regard remains.
The observed PFS was similar between the Asia subpopulation (HR, 0.64) and the global population (HR, 0.73); however, the smaller sample size and differences in follow-up suggest a cautious interpretation of differences observed between these populations. Despite IPI distribution being similar (supplemental Table 1), there were some observed differences that may reflect regional and biologic differences in DLBCL between these populations. Consistent with previously reported differences in cell-of-origin distribution seen in Asia vs Western populations,24,25 these trends were also observed in the present study. Although the clinical implication of the cell of origin remains limited outside of clinical trials,26 ∼50% of patients in the Asia subpopulation had the ABC subtype compared with approximately one-third of patients in the global population. In an exploratory subgroup analysis, inferior outcomes among patients with the ABC subtype who received R-CHOP was similar in both the Asia and global populations (data not shown). Similarly, the proportion of double-expressor lymphoma was slightly lower in the Asia subpopulation (∼30%) than in the global population (∼40%), whereas outcomes were also poor with R-CHOP.16 Other findings of interest include the relatively fewer patients diagnosed with the less common large B-cell lymphoma such as Epstein-Barr virus–positive DLBCL, high-grade B-cell lymphoma, not otherwise specified or high-grade B-cell lymphoma with rearrangements in MYC and BCL2 and/or BCL6 in the Asia subpopulation than the global population.
In terms of patient characteristics and disease presentation, patients in the Asia subpopulation were slightly younger than in the global population and were less likely to present with bulky disease, were more likely to present with elevated lactate dehydrogenase levels, and presented less frequently with extranodal disease. Whether these differences reflect biologic differences that can be identified in the assays evaluated (immunohistochemistry, Nanostring, fluorescence in situ hybridization) or other exploratory assays such as broader gene expression profiling or mutation analyses will be important to explore to develop better insights into ethnic and regional differences in DLBCL.
A key strength of this study is that it allowed analysis of a large cohort of Asia patients enrolled in the POLARIX global study and included patients from mainland China, Japan, Republic of Korea, and Taiwan where DLBCL is the most commonly observed non-Hodgkin lymphoma subtype. The study was performed in a double-blinded manner, which minimized the potential effects of experimenter bias and provided confidence regarding the similarities observed in the safety profiles of both treatment regimens. Despite the relatively short median follow-up period of 24.2 months in our study, the analysis was gated on a requisite number of PFS events, which was reached. In addition, the differences observed at the 24-month PFS and EFS milestones demonstrated a 7.7% and 8.6% increase, respectively, in the rate of patients who were free of events in the Pola-R-CHP arm compared with the R-CHOP arm, which, in the context of 24-month PFS and 24-month EFS,27,28 may predict a future survival advantage. Although an improvement in lymphoma survivorship is undoubtedly important, the current observations of PFS and EFS improvement with Pola-R-CHP and the similar safety profiles for both treatment regimens demonstrate the clinical benefit of replacing vincristine with polatuzumab vedotin within the R-CHOP regimen for previously untreated DLBCL. By experiencing fewer disease relapses, progressions, deaths, and subsequent antilymphoma treatments, patients treated with Pola-R-CHP have a higher rate of cure after 1L therapy.
In this double-blind trial, subsequent lymphoma therapy was also collected in a double-blind fashion. Patients in the R-CHOP arm received more systemic therapies than those in the Pola-R-CHP arm (27.9% vs 17.0%), which was associated with more PFS events and indicated a higher treatment burden. This trend was consistent between the Asia and global populations.16 Although more patients in the R-CHOP arm of the global population subsequently received autologous stem cell transplantation and chimeric antigen receptor T-cell therapy than those in the Pola-R-CHP arm,16 this difference was not evident in the Asia subpopulation, which may be related to factors specific to the Asia subpopulation analysis that go beyond patient fitness and eligibility for these therapies that apply to all patients with DLBCL globally. The shorter follow-up (median 24 months, but as low as 7 months) in the Asia subpopulation may have limited the number of patients eligible for these therapies. Furthermore, access to autologous stem cell transplantation in this region may have been further limited by the concurrent global COVID-19 pandemic. At the time of the study’s primary analysis, chimeric antigen receptor T-cell therapy was commercially unavailable in China, and was not broadly available in the other regions represented in the Asia subpopulation and only indicated for third-line-plus therapy.
In conclusion, results in the Asia subpopulation of POLARIX were consistent with those in the global population, with a comparable safety profile, and no new safety signals observed in Asia patients.
Acknowledgments
The authors thank the participating patients and their families, and the investigators, research nurses, study coordinators, and operations staff, as well as the Lymphoma Academic Research Organisation, and the Data and Safety Monitoring Committee.
This study was sponsored by Genentech, Inc and F. Hoffmann-La Roche Ltd. Third-party medical writing assistance, under the direction of the authors, was provided by Tracey McManus and Rachel Bell of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: All authors reviewed the data, confirmed the completeness and accuracy of the results and the study’s fidelity to the protocol and statistical analysis plan, reviewed the manuscript, and agreed on its submission for publication.
Conflict-of-interest disclosure: H.T. reports honoraria from Bristol Myers Squibb and Roche, consulting or an advisory role for Celgene/Bristol Myers Squibb, Incyte, and Roche, research funding from Roche/Genentech (institutional), and travel, accommodations, and expenses from Roche. S.R. reports speakers’ bureau fees from Chugai Pharma, Janssen, and Ono Pharmaceutical. H.G. reports speakers’ bureau fees from Chugai Pharma, Janssen, Kyowa Kirin, Novartis, MSD, and Daiichi Sankyo, and research funding from Bristol Myers Squibb. Y.T. reports speakers’ bureau fees from AbbVie, Celgene, Chugai Pharma, Eisai, Janssen, and Ono Pharmaceutical. T.M.K. reports consulting or advisory roles from AstraZeneca, Hanmi, Janssen, Novartis, Regeneron, Roche/Genentech, and Takeda, and research funding from AstraZeneca, Korea Health Industry Development Institute. X.C.-H.T. reports honoraria from Kirin Pharmaceuticals, Novartis, and Takeda, consulting or advisory role for Astellas Pharma, Chugai Pharma, Novartis, Pfizer, and Roche, and speakers’ bureau fees from Astellas Pharma, Bristol Myers Squibb, Celgene, Harvester Trading Co, Janssen, Kirin Pharmaceuticals, Novartis, Pfizer, Roche, and Takeda. H.K. reports research funding from Chugai Pharmaceutical and Takeda Pharmaceutical, and honoraria from Novartis and Takeda Pharmaceutical. K.A. reports research funding from Solasia, Novartis, Janssen, Otsuka, IQVIA, Zenyaku, Chugai, and Astellas, and honoraria from Kyowa Kirin, Takeda, Chugai, Meiji Seika, Eisai, and Mochida. J.P.S. reports consulting or advisory role for AbbVie, Acerta Pharma/AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Merck, and TG Therapeutics, and research funding from Acerta Pharma (institutional), Celgene (institutional), Genentech (institutional), Gilead Sciences (institutional), Merck (institutional), Pharmacyclics (institutional), Seattle Genetics (institutional), Takeda (institutional), and TG Therapeutics (institutional). L.H.S. reports consulting for Novartis, Genmab, Debiopharm, Teva, Roche/Genentech, AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Takeda, TG Therapeutics, and Verastem, and research funding from Teva and Roche/Genentech. L.B. is an employee of F. Hoffmann La Roche Ltd. X.W. is an employee of F. Hoffmann La Roche Ltd, and receives F. Hoffmann-La Roche Ltd stocks/stock options. Y.J., J.H., and C.L. are employees of Genentech, Inc, and receive F. Hoffmann-La Roche Ltd stocks/stock options. K.I. reports honoraria from AbbVie, Allergan, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Fujifilm, HUYA Bioscience International, Janssen, Kyowa Kirin, MSD, Mundipharma, Novartis, Ono Pharmaceutical, and Takeda, consulting or advisory role for AbbVie, AstraZeneca, Bayer, Celgene, Kyowa Kiri, and Ono Pharmaceutical, and research funding for Chugai Pharma and Eisai. The remaining authors declare no competing financial interests.
Correspondence: Jun Zhu, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Fucheng Rd No 52, Beijing 100142, China; e-mail: zhujun3346@163.com.
References
Author notes
∗All authors contributed equally to this study.
Presented in abstract form at the 2022 annual meeting of the American Society of Clinical Oncology, Chicago, 3 June 2022 to 7 June 2022, followed by a subsequent encore at the European Hematology Association 2022 Congress, Vienna, 9 June 2022 to 17 June 2022. In addition, the results were presented in abstract form at the 2022 annual meeting of the Chinese Society of Clinical Oncology, Xiamen, China, 5 November 2022 to 12 November 2022.
For eligible studies, qualified researchers may request access to individual patient-level clinical data through the clinical study data request platform. At the time of writing this request, the platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, available at: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked owing to a potential increase in risk of patient reidentification.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal