Key Points
NSAIDs can normalize hematologic parameters and serum inflammatory markers in patients with Ghosal hematodiaphyseal dysplasia syndrome.
Prostaglandins and leukotrienes are aberrantly augmented in patients with Ghosal syndrome and are responsive to treatment with NSAIDs.
Abstract
Advances in genomic diagnostics hold promise for improved care of rare hematologic diseases. Here, we describe a novel targeted therapeutic approach for Ghosal hematodiaphyseal dysplasia, an autosomal recessive disease characterized by severe normocytic anemia and bone abnormalities due to loss-of-function mutations in thromboxane A synthase 1 (TBXAS1). TBXAS1 metabolizes prostaglandin H2 (PGH2), a cyclooxygenase (COX) product of arachidonic acid, into thromboxane A2. Loss-of-function mutations in TBXAS result in an increase in PGH2 availability for other PG synthases. The current treatment for Ghosal hematodiaphyseal dysplasia syndrome consists of corticosteroids. We hypothesize that nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit COX-1 and COX-2, could ameliorate the effects of TBXAS1 loss and improve hematologic function by reducing prostaglandin formation. We treated 2 patients with Ghosal hematodiaphyseal dysplasia syndrome, an adult and a child, with standard doses of NSAIDs (aspirin or ibuprofen). Both patients had rapid improvements concerning hematologic parameters and inflammatory markers without adverse events. Mass spectrometry analysis demonstrated that urinary PG metabolites were increased along with proinflammatory lipoxygenase (LOX) products 5-hydroxyeicosatetraenoic acid and leukotriene E4. Our data show that NSAIDs at standard doses surprisingly reduced both COX and LOX products, leading to the resolution of cytopenia, and should be considered for first-line treatment for Ghosal hematodiaphyseal dysplasia syndrome.
Introduction
The growing use of genomic diagnostics holds promise for tailored therapies for rare hematologic disorders. In this study, we describe a treatment that reverses the effects of the underlying genetic abnormality in Ghosal hematodiaphyseal dysplasia syndrome, an autosomal recessive disease caused by inherited loss-of-function mutations in thromboxane A synthase 1 (TBXAS1).1,2
Ghosal hematodiaphyseal dysplasia syndrome is characterized by corticosteroid-sensitive normocytic anemia and increased bone density along with diaphyseal dysplasia.1,2 Thirty-six cases of Ghosal hematodiaphyseal dysplasia syndrome of varying severities have been reported, most commonly in Middle Eastern, North African, and South Asian individuals; recently, patients who are of North American-European descent have also been identified. Ghosal hematodiaphyseal dysplasia syndrome has been traditionally treated using repeated courses of corticosteroids to maintain adequate hemoglobin levels.1-12
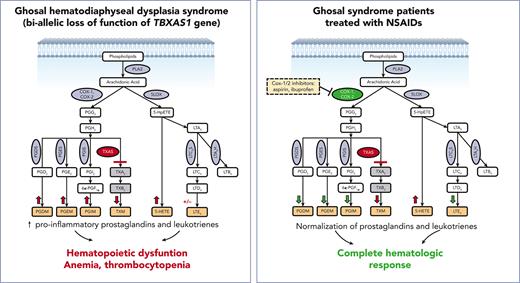
Because disruption of TBXAS1 causes accumulation of arachidonic acid (AA) metabolites due to the rediversion of its prostaglandin H2 (PGH2) substrate (Figure 1A), we hypothesized that nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase-1 (COX-1) and COX-2, could ameliorate the effects of TBXAS1 loss and improve the hematologic function by reducing prostaglandin formation. We administered ibuprofen or aspirin, at doses that inhibit both COX-1 and COX-2, to 2 patients, an adult and a child, with Ghosal hematodiaphyseal dysplasia syndrome. Our data show that COX-1/2 inhibition improves hematologic and inflammatory parameters and should be considered as a first-line treatment for Ghosal hematodiaphyseal dysplasia syndrome.
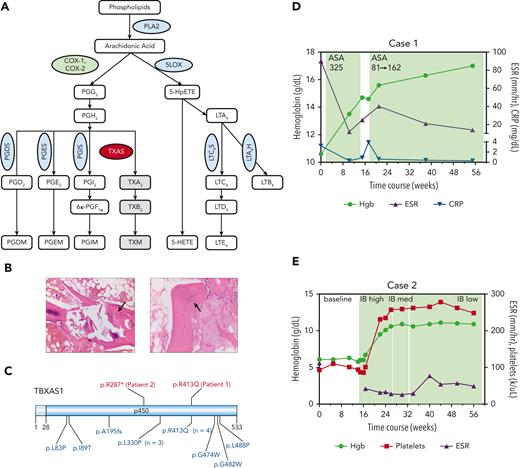
AA metabolism and the clinical characteristics of 2 patients with Ghosal hematodiaphyseal dysplasia syndrome. (A) A schematic diagram of the AA metabolism pathway. AA is released from the membrane phospholipids by PLA2 and is metabolized by COX-1 and −2 enzymes, (green oval) first into the unstable intermediate metabolite PGG2, which is converted to PGH2. PGH2 serves as a substrate for several PG synthases (PGDS, PGES, PGIS, blue ovals) that generate the following proinflammatory PG metabolites: PGD2, PGE2, and PGI2. PGH2 can also be converted to TXA2 through the action of thromboxane synthase (red oval), the gene which is mutated in Ghosal hematodiaphyseal dysplasia syndrome. In addition to the COX-1/2 enzymes, AA is metabolized by 5-LOX (blue oval) to generate various products of hydroperoxyeicosatetraenoic acid, including 5-HETE and leukotrienes, for example, LTE4. (B) Bone marrow biopsy sections for patient 1 have aspiration artifact but show an apparent reduction in marrow cellularity out of proportion to peripheral counts. On the left, H&E-stained section at an original magnification ×10 additionally shows thickened trabeculae (black arrow), and on the right, the H&E-stained section at an original magnification ×20 highlights disorganized osteocytes (black arrow). (C) A schematic diagram of thromboxane synthase A 1, encoded by the TBXAS1, showing the locations of the variants in the 2 patients reported in this manuscript (shown in red, above the diagram), and those from 13 sequenced cases previously reported in the literature (shown in blue, below the diagram). Diagram created using Domain Graph (DOG, version 2.0). D (for Case 1) and E (for Case 2), show. H&E, hematoxylin and eosin; 5-LOX, 5-lipooxygenase; PLA2, phospholipase A2; TXA2, thromboxane A2.
AA metabolism and the clinical characteristics of 2 patients with Ghosal hematodiaphyseal dysplasia syndrome. (A) A schematic diagram of the AA metabolism pathway. AA is released from the membrane phospholipids by PLA2 and is metabolized by COX-1 and −2 enzymes, (green oval) first into the unstable intermediate metabolite PGG2, which is converted to PGH2. PGH2 serves as a substrate for several PG synthases (PGDS, PGES, PGIS, blue ovals) that generate the following proinflammatory PG metabolites: PGD2, PGE2, and PGI2. PGH2 can also be converted to TXA2 through the action of thromboxane synthase (red oval), the gene which is mutated in Ghosal hematodiaphyseal dysplasia syndrome. In addition to the COX-1/2 enzymes, AA is metabolized by 5-LOX (blue oval) to generate various products of hydroperoxyeicosatetraenoic acid, including 5-HETE and leukotrienes, for example, LTE4. (B) Bone marrow biopsy sections for patient 1 have aspiration artifact but show an apparent reduction in marrow cellularity out of proportion to peripheral counts. On the left, H&E-stained section at an original magnification ×10 additionally shows thickened trabeculae (black arrow), and on the right, the H&E-stained section at an original magnification ×20 highlights disorganized osteocytes (black arrow). (C) A schematic diagram of thromboxane synthase A 1, encoded by the TBXAS1, showing the locations of the variants in the 2 patients reported in this manuscript (shown in red, above the diagram), and those from 13 sequenced cases previously reported in the literature (shown in blue, below the diagram). Diagram created using Domain Graph (DOG, version 2.0). D (for Case 1) and E (for Case 2), show. H&E, hematoxylin and eosin; 5-LOX, 5-lipooxygenase; PLA2, phospholipase A2; TXA2, thromboxane A2.
Study design
After informed consent was obtained, patients were treated with ibuprofen or aspirin as per routine clinical care, independent of each other, at the discretion of their respective treating physicians. Because aspirin is associated with Reye syndrome among children, the pediatric patient received ibuprofen. Eicosanoid analysis from patients and control participants was performed as a part of the ongoing institutional review board–approved studies at the University of Pennsylvania.
Whole exome sequencing (WES) of peripheral blood DNA was performed at clinical genetic testing laboratories (Genome Diagnostics Nijmegen Maastricht, Nijmegen, Netherlands; and Invitae, San Francisco, CA).
Mass spectrometry of urinary eicosanoid metabolites, which enables reliable quantitative analysis of systemic eicosanoid formation in vivo,13 was performed using several 24-hour urine collection samples, collected at baseline (for patient 2) and after 3 months of high-dose NSAID treatment, after withholding NSAID for 14 days, and after 3 weeks of low-dose NSAID (for both patients). Results were analyzed using analysis of variance (ANOVA) with a significant P-value < .05 (see supplemental Material, available on the Blood website).
Results and discussion
Case presentations and genetic diagnosis
Patient 1
A 30-year-old Pakistani male who was born to a consanguineous family was evaluated for life-long normocytic anemia and thrombocytopenia. He had chronic fatigue, joint stiffness, and diarrhea. A bone marrow biopsy at 2 years of age was nondiagnostic. He received ∼50 red blood cell transfusions before the age of 7 years. Corticosteroids produced periods of transfusion independence; however, the anemia relapsed after steroid tapering. Two of the patient’s siblings died in infancy; 1 of them had a condition resembling his, and the other died of an unspecified neurological disorder.
Laboratory studies revealed normocytic anemia (hemoglobin, 8.5-10.6 g/dL), platelets of 100 to 120 × 103/μL, and intermittent mild neutropenia with absolute neutrophil count of ∼1.2-2.5 × 103/μL. Erythrocyte sedimentation rate (ESR) and C-reactive protein level were persistently elevated at 76-111 mm/h and 2.1-3.2 mg/dL, respectively. The erythropoietin level was >741 miU/mL (normal range, 2.6-18.5). Iron levels were normal. Reticulocytes ranged from 88 to 109 × 103/μL (reticulocyte index <2), consistent with hypoproliferative anemia. Two bone marrow biopsies, both technically challenging, showed reduced hematopoietic elements, with orderly normoblastic maturation of evaluable scant erythroid precursors as well as thickened trabeculae and disorganized osteocytes (Figure 1B). Lymphocyte telomere lengths were short for the given age, and chromosome breakage was normal. WES helped identify a likely pathogenic homozygous p.R413Q missense substitution in TBXAS1 (Figure 1C).2 The skeletal survey showed cortical thickening of bilateral femoral shafts and proximal humeri, with diffuse osseous sclerosis throughout the spine. Bone density was increased (lumbar spine [LS] L1-L4; Z-score +1.2), consistent with that observed in Ghosal hematodiaphyseal dysplasia syndrome.1
Patient 2
A previously healthy 3-year-old girl of Filipino descent was found to have severe anemia (hemoglobin, 5.3 g/dL) and thrombocytopenia (platelets, 12 × 103/μL) during hospitalization for bacterial pneumonia. Normocytic anemia (hemoglobin, 6.0-7.5mg/dL) and thrombocytopenia (platelet range, 60-110 × 103/μL) persisted after resolution of infection, with intermittent neutropenia with the absolute neutrophil count as low as 0.19 × 103/μL. The reticulocyte count was 34 × 103/μL (reticulocyte index <2). The ESR was markedly elevated to between 112 and >130 mm/h. Bone marrow was profoundly hypocellular, with fatty replacement and areas of fibrotic stroma containing scattered hematopoietic elements. The karyotype was normal. Paroxysmal nocturnal hemoglobinuria screen, hemoglobin electrophoresis, chromosomal breakage studies, and lymphocyte telomere lengths were unremarkable. Via WES, we identified a novel, homozygous, likely pathogenic p.R287∗ TBXAS1 variant, inherited from her clinically unaffected parents, each heterozygous for the variant. Bone density was markedly increased (LS, L1-L4; Z-score +2.4), consistent with that observed in Ghosal hematodiaphyseal dysplasia syndrome.
Dose-dependent hematologic response and eicosanoid suppression by COX-1/2 inhibitors
Patient 1 was started on aspirin with a dose of 325 mg per day, with normalization of hematologic and inflammatory parameters and improvements in fatigue and joint stiffness after 2 months (Figure 1D). When aspirin was discontinued for 14 days, symptoms recurred and were accompanied by a rise in the C-reactive protein level. Resumption of aspirin at a dose of 81 mg per day (a dose that preferentially targets COX-1) maintained blood counts; however, joint stiffness persisted and ESR remained elevated. An intermediate aspirin dose (162 mg per day) normalized inflammatory markers and improved fatigue and joint stiffness. Sixteen months after the initiation of therapy, the patient maintained normal blood counts and inflammatory markers on a daily dose of 162 mg of aspirin.
Patient 2 had a rapid improvement of hemoglobin, platelet count, and ESR within 1 month of receiving ibuprofen at a dose of 30 mg/kg per day, given in divided doses (Figure 1E). To reduce the risk of potential adverse events, the ibuprofen dose was reduced to 10 mg/kg per day. Nineteen months after initiation of therapy, the patient maintained hemoglobin level at 10.6-11.5 g/dL and ESR at 40 to 60 mm/h.
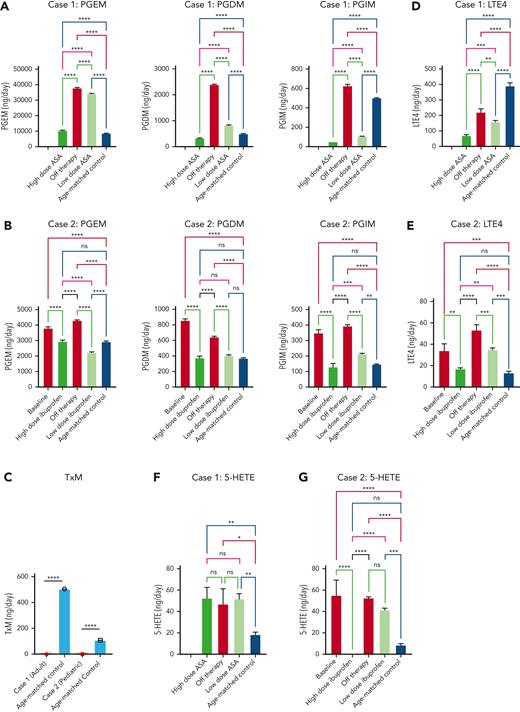
Because loss of TBXAS1 leads to the accumulation of its upstream substrate PGH2, which can be processed by synthases to other PGs (Figure 1A), our findings of absent thromboxane A2 metabolite and increased accumulation of downstream urinary PG metabolites PG D metabolite (PGDM), PGEM, and PGIM in both patients could be expected (Figure 2A-C). The response to NSAIDs, which inhibit PGH2 formation by inhibiting COX upstream, implies that patients with Ghosal hematodiaphyseal dysplasia syndrome also have an accumulation of the upstream AA substrate. Increased AA, in turn, can augment biosynthesis of not only the proinflammatory PGs PGE2, PGD2, and PGI2 but also of the proinflammatory lipoxygenase products, reflected by 5-hydroxyeicosatetraenoic acid(5-HETE) and leukotriene E4 (LTE4) (Figure 2D-G). The higher doses of the NSAIDs also reduced biosynthesis of the lipoxygenase products of AA along with the PGs, normalizing hematologic and inflammatory parameters. Lower doses of aspirin and ibuprofen showed a less impressive reduction of the augmented eicosanoids, consistent with their less effective inhibition of COX-2, the dominant source of inflammatory PG formation.
Mass spectrometry analysis of 24-hour urinary metabolites performed throughout therapy for 2 patients with Ghosal hematodiaphyseal dysplasia syndrome. Measurements of the urinary prostaglandin metabolites PGEM, PGDM, and PGIM are shown in panels A (for patient 1) and B (for patient 2). (C) The urinary metabolite of thromboxane A2 (TXM, 11-dehydrothromboxane B2 [9α,15S-dihydroxy-11-oxothromba-5Z,13E-dien-1-oic acid]). No TXM signal was detected in the patients’ urine samples. Daily productions of TXM in adult and child healthy controls were 504 ± 1 and 107 ± 3 ng/d, respectively. The urinary levels of LTE4 are shown in panels D for case 1 and E for case 2. The urinary levels of 5-HETE are in panels F for case 1 and G for case 2. In each plot, the bar represents a mean ± standard deviation, for 3 replicate measurements, with statistical analysis indicated by the bar above, performed by one-way ANOVA. Red bars show urinary metabolites that were measured off therapy. Green bars show the metabolite levels on COX1/2 inhibitor therapy. Urinary metabolites for a control subject, age-matched for each patient are shown by the blue bars. (H) The results of eicosanoid metabolite analysis from A-F are summarized in the schematic diagram of AA metabolism, showing the aberrant accumulation of PGDM, PGEM, PGIM, 5-HETE and LTE4 in Ghosal hematodiaphyseal dysplasia syndrome (upward red arrows). In Ghosal hematodiaphyseal dysplasia syndrome, TBXAS1 is inactive (red line), preventing the conversion of PGH2 to TXA2 and downstream conversion to TXB2 and TXM. (I) Inhibition of COX-1/2 by NSAIDs (yellow box). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Mass spectrometry analysis of 24-hour urinary metabolites performed throughout therapy for 2 patients with Ghosal hematodiaphyseal dysplasia syndrome. Measurements of the urinary prostaglandin metabolites PGEM, PGDM, and PGIM are shown in panels A (for patient 1) and B (for patient 2). (C) The urinary metabolite of thromboxane A2 (TXM, 11-dehydrothromboxane B2 [9α,15S-dihydroxy-11-oxothromba-5Z,13E-dien-1-oic acid]). No TXM signal was detected in the patients’ urine samples. Daily productions of TXM in adult and child healthy controls were 504 ± 1 and 107 ± 3 ng/d, respectively. The urinary levels of LTE4 are shown in panels D for case 1 and E for case 2. The urinary levels of 5-HETE are in panels F for case 1 and G for case 2. In each plot, the bar represents a mean ± standard deviation, for 3 replicate measurements, with statistical analysis indicated by the bar above, performed by one-way ANOVA. Red bars show urinary metabolites that were measured off therapy. Green bars show the metabolite levels on COX1/2 inhibitor therapy. Urinary metabolites for a control subject, age-matched for each patient are shown by the blue bars. (H) The results of eicosanoid metabolite analysis from A-F are summarized in the schematic diagram of AA metabolism, showing the aberrant accumulation of PGDM, PGEM, PGIM, 5-HETE and LTE4 in Ghosal hematodiaphyseal dysplasia syndrome (upward red arrows). In Ghosal hematodiaphyseal dysplasia syndrome, TBXAS1 is inactive (red line), preventing the conversion of PGH2 to TXA2 and downstream conversion to TXB2 and TXM. (I) Inhibition of COX-1/2 by NSAIDs (yellow box). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Taken together, our results indicate that full or intermediate-dose NSAIDs, at doses inhibitory to COX-2, are most effective at normalizing both hematologic parameters and inflammatory markers. Low-dose NSAIDs are sufficient for clinical hematologic improvement but do not fully inhibit excess eicosanoid accumulation and do not normalize inflammatory markers. Future studies are needed to determine whether complete inhibition of elevated eicosanoids with full-dose NSAIDs improves long-term patient outcomes compared with partial eicosanoid inhibition with lower-dose NSAIDs.
Much literature exists regarding the effect of eicosanoids on hematopoiesis, with the role of individual metabolites in hematopoiesis incompletely defined, with some evidence for opposing effects of PGs on stem cells and progenitor cells.14-18 Although our study does not identify the precise locus of eicosanoid effects on hematopoietic production in the Ghosal hematodiaphyseal dysplasia syndrome, our results demonstrate that impaired hematopoiesis and various cytopenia in patients with Ghosal hematodiaphyseal dysplasia syndrome were likely mediated by prostaglandins and leukotrienes and were entirely reversed by NSAIDs. The most commonly reported bone marrow disorders in Ghosal hematodiaphyseal dysplasia syndrome are hypocellularity, with reduced but normally maturing erythroid lineage, and marrow fibrosis.19,20 The hematopoietic dysfunction in Ghosal hematodiaphyseal dysplasia syndrome is thus reminiscent of autoimmune myelofibrosis, which can occur in inflammatory conditions, and results in hypoproliferative anemia that is typically responsive to corticosteroids.21
Pharmacological inhibitors of TBXAS1 were developed to treat thrombosis, but their efficacy was limited by the accumulation of PGH2, which can also activate the thromboxane receptor.22-24 The combination of TBXAS1 inhibitors with thromboxane receptor antagonists blunts this effect, revealing an antithrombotic efficacy that is augmented by increased formation of the platelet inhibitor, PGI2. In line with our observations in Ghosal hematodiaphyseal dysplasia syndrome, aspirin abolished the antithrombotic efficacy of this drug combination in dogs, consistent with TBXAS1 inhibition causing the accumulation of not merely PGH2 but also AA.25 In patients treated with TBXAS1 inhibitors, cytopenia analogous to Ghosal hematodiaphyseal dysplasia syndrome have not been reported.
In conclusion, our study shows that higher-dose NSAIDs can normalize hematologic parameters and serum inflammatory markers in patients with Ghosal hematodiaphyseal dysplasia syndrome. Our results demonstratefor the first time, to the best of our knowledge, that eicosanoid biosynthesis, which spans both PG and leukotriene formation, is augmented in patients with Ghosal hematodiaphyseal dysplasia syndrome and is responsive to treatment with aspirin and ibuprofen. Our data suggest that NSAIDs, which have a favorable side-effect profile compared with corticosteroids, should be considered as a first-line treatment for Ghosal hematodiaphyseal dysplasia syndrome.
Acknowledgments
The authors thank the patients and their families for participating in this study.
This work was supported by the National Institutes of Health (grant T32CA009679 and L30CA274783) (T.J.B.), (grant R03HL160678-01), and (American Society of Hematology Scholar Awards) (D.V.B.). G.A.F. is the McNeil Professor of Translational Medicine and Therapeutics.
Authorship
Contribution: T.J.B. and N.B. analyzed clinical data; H.M. and E.R. performed mass spectroscopy analyses; H.M. performed statistical analyses of mass spectroscopy results; C.M. and A.D. participated in patient care and contributed critical insights for their diagnosis and management and performed critical literature review; J.Q. analyzed bone marrow histology; G.A.F. initiated the collaboration between the 2 centers, and analyzed and contributed critical insights for eicosanoid metabolite analysis; M.C. and D.V.B. oversaw the clinical care, diagnostic work-up, treatment of the patients, and the study; T.J.B. and D.V.B. synthesized all data and wrote the manuscript; and all authors edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Melanie Cotter, Children's Health Ireland at Temple Street/Crumlin, Dublin, Ireland; e-mail: melanie.cotter@cuh.ie; and Daria V. Babushok, Biomedical Research Building, Room 808, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: daria.babushok@pennmedicine.upenn.edu.
References
Author notes
∗T.J.B., N.B., and H.M. contributed equally to this study.
†M.C. and D.V.B. contributed equally to this study.
Data are available on request from the corresponding authors, Melanie Cotter (melanie.cotter@cuh.ie) and Daria V. Babushok (daria.babushok@pennmedicine.upenn.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Mass spectrometry analysis of 24-hour urinary metabolites performed throughout therapy for 2 patients with Ghosal hematodiaphyseal dysplasia syndrome. Measurements of the urinary prostaglandin metabolites PGEM, PGDM, and PGIM are shown in panels A (for patient 1) and B (for patient 2). (C) The urinary metabolite of thromboxane A2 (TXM, 11-dehydrothromboxane B2 [9α,15S-dihydroxy-11-oxothromba-5Z,13E-dien-1-oic acid]). No TXM signal was detected in the patients’ urine samples. Daily productions of TXM in adult and child healthy controls were 504 ± 1 and 107 ± 3 ng/d, respectively. The urinary levels of LTE4 are shown in panels D for case 1 and E for case 2. The urinary levels of 5-HETE are in panels F for case 1 and G for case 2. In each plot, the bar represents a mean ± standard deviation, for 3 replicate measurements, with statistical analysis indicated by the bar above, performed by one-way ANOVA. Red bars show urinary metabolites that were measured off therapy. Green bars show the metabolite levels on COX1/2 inhibitor therapy. Urinary metabolites for a control subject, age-matched for each patient are shown by the blue bars. (H) The results of eicosanoid metabolite analysis from A-F are summarized in the schematic diagram of AA metabolism, showing the aberrant accumulation of PGDM, PGEM, PGIM, 5-HETE and LTE4 in Ghosal hematodiaphyseal dysplasia syndrome (upward red arrows). In Ghosal hematodiaphyseal dysplasia syndrome, TBXAS1 is inactive (red line), preventing the conversion of PGH2 to TXA2 and downstream conversion to TXB2 and TXM. (I) Inhibition of COX-1/2 by NSAIDs (yellow box). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/13/10.1182_blood.2022018667/7/m_blood_bld-2022-018667-gr2hi.jpeg?Expires=1770841023&Signature=D~vJ0Z5qrP2EK2hPaKoHX8YdwY8wihfFf-~IG6gbjer4P13FsYwehTwvbI96jji11aXDyCKiUfyNgJxUTi3X3sDLoq3iwjVj3nr5ev3NMfR614P9HaXJwcY8T8yUXB8rUIT~R0ljPxG5J869M1KCn6Yvapbwn0pveQ7zjmVSGMapnyKg8pPYhJt5JLCwhl3~sgdgdTo9Fw-K1rQIXM5kmofGIdMJjfCaRs6GS2acX4DgpEto6opnbY4BbjeEY4OjjEGvTQnFRhGE1V2KbQpw8ku-z8BkMk9xnIjoSLZ-MYk460GGIAwVenx~ciBwwKvPpMHP8AvMpwDnuNnGeBgQdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal