In this issue of Blood, Brown et al show that novel therapy with intermediate- to high-dose nonsteroidal antiinflammatory drugs (NSAIDs) overcomes augmented inflammatory eicosanoid production and can completely restore hematopoiesis in patients with Ghosal hematodiaphyseal dysplasia (GHDD), an ultrarare inherited bone marrow failure syndrome due to mutations in thromboxane A synthase 1 (TBXAS1).1
GHDD, also known as Ghosal syndrome, is an autosomal recessive disorder, typically featuring corticosteroid responsive myelophthisic anemia and characteristic bony changes with cortical thickening of diaphyses on imaging.2 The exact mechanism underlying the distinctive hematopoietic impairment in GHDD is not well understood. In the absence of thromboxane activity, more prostaglandin H2 is available for other prostaglandin synthases and results in accumulation of their products. It is hypothesized that these downstream pro-inflammatory prostaglandin and leukotriene metabolites may cause direct injury to the bone marrow and lead to remodeling of the cortices.3 The incidence of GHDD has not been formally evaluated, with fewer than 40 cases reported worldwide. These mostly originate in North Africa, the Middle East, and Southeast Asia. Recently, novel TBXAS1 mutations have been described in a White family in North America.4 Patient 2 in the study by Brown et al is the first reported case of GHDD in a child of Filipino descent, underscoring that many more cases are likely to surface as more sophisticated genetic testing, including whole exome and genome sequencing, is used for the diagnosis of variable presentations of this disorder.
Patients typically present in childhood with characteristic bony changes, persistent normocytic anemia often requiring transfusion support, and evidence of underlying inflammation with elevations in erythrocyte sedimentation rate and C-reactive protein. Bone marrow examination often reveals hypocellularity and, in some cases, myelofibrosis or decreased erythroid precursors.3 Historically, treatment with corticosteroids has been empirically used.5 This is usually given in short courses titrated to the patient’s response or, in refractory cases, maintained on low doses indefinitely.3
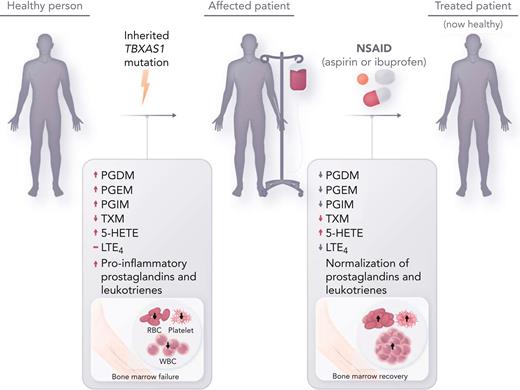
This initial report by Brown et al seeks to bridge the gap between the molecular understanding of the genetic driver underlying GHDD with targeted therapeutics. The authors hypothesized that inhibition of cyclooxygenase-1 (COX-1) and COX-2 with NSAIDs would reduce prostaglandin formation and thereby improve hematopoiesis. One adult patient (patient 1) and 1 pediatric patient (patient 2) were treated with aspirin and ibuprofen, respectively, owing to the risk of Reye syndrome with aspirin in the pediatric population. Hematologic reassessment and mass spectrometry of urinary eicosanoid metabolites were performed at baseline (in patient 2), after 3 months of high-dose therapy, after a 14-day drug holiday, and after 3 weeks of low-dose therapy. The results of the treatment were striking (see figure). Both patients had total resolution of systemic symptoms, hematologic abnormalities, and inflammatory markers at high dose levels (aspirin 325 mg and ibuprofen 30 mg/kg daily). Discontinuation of therapy led to recurrence of anemia and systemic symptoms. Resumption of therapy at low dose (aspirin 81 mg) improved hematopoiesis but failed to correct inflammatory markers or systemic symptoms. At intermediate doses (aspirin 162 mg and ibuprofen 10 mg/kg daily), both patients remain symptom free and have become completely independent of transfusion therapy over long-term follow-up. Moreover, Brown et al demonstrate for the first time that downstream COX and lipoxygenase (LOX) products were aberrantly amplified in patients with Ghosal syndrome and, interestingly, that treatment with NSAIDs not only reduced downstream COX products but also reduced LOX products. Their study reveals that eicosanoid suppression by NSAIDs appears to occur in a dose-dependent fashion. These findings also correlate with the patients’ clinical responses to intermediate and high-dose therapy, which would more effectively inhibit COX-2 compared with lower-dose therapy. Furthermore, the resolution of hematologic abnormalities despite continued inflammation with low dosing of NSAIDs implies that 1 or more of the prostaglandins likely have a direct influence on hematopoiesis independent of their inflammatory effect.
Inherited thromboxane A synthase 1 (TBXAS1) mutations cause GHDD, which leads to enlargement of the long bone diaphysis and bone marrow failure. Affected patients often require frequent transfusion support, and empiric treatment with steroids is sometimes effective. Treatment with NSAIDs (aspirin and ibuprofen) at intermediate to high doses, which inhibits COX-1 and COX-2, reverse aberrant prostaglandin and leukotriene metabolite production in GHDD, leading to restored hematopoiesis. RBC, red blood cell; WBC, white blood cell.
Inherited thromboxane A synthase 1 (TBXAS1) mutations cause GHDD, which leads to enlargement of the long bone diaphysis and bone marrow failure. Affected patients often require frequent transfusion support, and empiric treatment with steroids is sometimes effective. Treatment with NSAIDs (aspirin and ibuprofen) at intermediate to high doses, which inhibits COX-1 and COX-2, reverse aberrant prostaglandin and leukotriene metabolite production in GHDD, leading to restored hematopoiesis. RBC, red blood cell; WBC, white blood cell.
This brief report furthers our understanding of hematopoietic suppression that occurs in inflammatory states at large. It specifically highlights the pathophysiologic consequences of abnormal eicosanoid accumulation in patients with GHDD and confirms prior hypotheses. Most importantly, these data provide compelling evidence for intermediate- to high-dose NSAID therapy as first-line treatment for these patients. Corticosteroids are known to inhibit phospholipase A2,6 which explains their therapeutic effect in this disorder. However, NSAIDs offer a proximal biologically targeted treatment approach with a more favorable side effect profile. Considering that the anticipated length of therapy in these patients tends to be prolonged, these characteristics make NSAIDs an attractive choice for frontline therapy over current standard of care. A reasonable approach in adults could start with high-dose aspirin 325 mg until complete response is achieved, with subsequent titration to the lowest dose needed to maintain clinical response. In the pediatric population, a similar strategy with weight-based ibuprofen dosing could be used. Further studies are needed, however, to identify how eicosanoids affect hematopoiesis, which cell(s) they affect the most, and whether inhibition of a single eicosanoid could be therapeutic in GHDD. Additionally, it would be important to investigate whether eicosanoids are implicated in other bone marrow failure states with inflammatory components, including genetic conditions such as Fanconi anemia.7
With the advent of whole exome and genome sequencing and their increasing use in the diagnostic landscape of hematologic and multisystem disorders, studies like this will become increasingly common. These cases emphasize how molecular understanding of a specific genetic disorder could allow for the repurposing of benign, widely available therapeutic agents to improve the standard of care in an ultrarare disease.
Conflict-of-interest disclosure: S.S. participates in a scientific advisory board for Sanofi-Genzyme. G.S declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal