More than 90% of the general population show evidences of past infection of Epstein-Barr virus (EBV). So why do only a few of us experience EBV-related disease? In this issue of Blood, Vietzen et al address this question in the context of EBV-associated infectious mononucleosis (IM or glandular fever) and EBV-associated posttransplant lymphoproliferative disorders (EBV+ PTLDs).1
For individual cases, functional CD8+ T-cell and natural killer (NK) cell assays can identify novel disorders with EBV involvement, thereby establishing the correct diagnosis and treatment.2,3 At a population level, polymorphisms in the classical HLA class I molecule HLA-A are associated with an increased risk of developing IM and EBV-associated classical Hodgkin lymphoma (EBV+ cHL).4,5 Mechanistic studies suggest that the association is due to the impact of classical HLA class I molecules on the established hierarchy of CD8+ T-cell responses against EBV latency II protein LMP1, and that polymorphisms in EBV latency genes influence the potency of CD8+ T-cell responses in EBV+ PTLD.6,7
HLA-E is a minimally polymorphic nonclassical HLA class I molecule that is highly conserved in European populations and is essentially restricted to only 2 alleles (E∗01:01. and E∗01:03) that appear to be evenly distributed. The alleles differ by a single amino acid that modifies the molecular function and level of cell-surface expression. Notably, HLA-E expression is retained in EBV+ PTLD– and EBV+ AIDS–related lymphomas.8 There are several lines of evidence suggesting that HLA-E may be involved in the pathogenesis of EBV-related diseases. HLA-E∗01:01 is a protective genetic factor in EBV+ cHL that is independent of the HLA-A allele status. It is also known that there are expanded populations of CD8bright T cells that recognize HLA-E in the context of the EBV-lytic protein BZLF1 in patients with the EBV-related disease, multiple sclerosis (MS).9
HLA-E has a role in both the innate and adaptive immune responses through its recognition by the T-cell receptor and the NK cell inhibitory CD94/NKG2A receptor, respectively. The CD94/NKG2A receptor is predominantly expressed in CD56bright NK cells (relative to CD56dim NK cells) and is expressed on a subset of activated CD8+ T cells. A restricted set of distinct EBV-encoded BZLF1 or LMP-1–derived peptides are presented by HLA-E, and specific LMP1 polymorphisms preferentially bind to HLA-E via NKG2A to block NK cell effector function.10
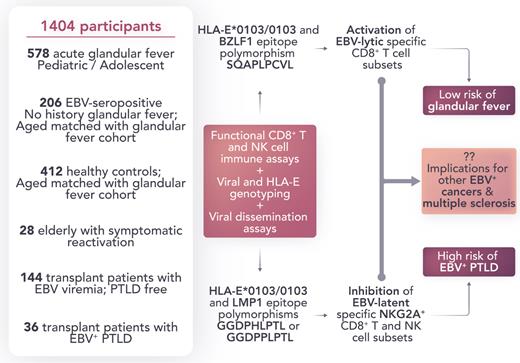
Vietzen et al postulated that variations in CD94/NKG2A–HLA-E interactions on T- and NK cell subsets might have functional relevance in EBV control. In an elegant study, they combined genetic association approaches with EBV dissemination and functional immune (CD8+ T- and NK) cell subset assays (see figure). The authors demonstrated that HLA-E–restricted immune responses and EBV epitope polymorphisms have a substantial impact on mediating susceptibility and protection from IM and EBV+ PTLD. Not only is their investigation notable for its scale (1404 participants; including those with IM, age-matched asymptomatic EBV carriers, elderly patients with symptomatic reactivation, transplant, and PTLD), but it is also distinguished by providing an immuno-mechanistic basis to back up their genetic association observations.
Role of host and viral genotype on susceptibility to IM and EBV+ PTLD. The development of IM depends on the host HLA-E allele and HLA-E–restricted CD8+ T-cell response. EBV+ PTLD are associated with the HLA-E/LMP-1/NKG2A axis and depend on specific EBV and host genetic variations involved in this pathway. Professional illustration by Somersault18:24.
Role of host and viral genotype on susceptibility to IM and EBV+ PTLD. The development of IM depends on the host HLA-E allele and HLA-E–restricted CD8+ T-cell response. EBV+ PTLD are associated with the HLA-E/LMP-1/NKG2A axis and depend on specific EBV and host genetic variations involved in this pathway. Professional illustration by Somersault18:24.
Collectively, the work of Vietzen et al shows that the development of IM depends on the host HLA-E allele and HLA-E–restricted CD8+ T-cell responses. The risk for PTLD is associated with the HLA-E/LMP-1/NKG2A axis and depends on specific EBV and host genetic variations involved in this pathway. They build upon previous foundational studies to provide new insight into the immunopathogenesis of IM and EBV+ PTLD. Further studies will be required to determine whether similar mechanisms that affect viral dissemination are applicable to other EBV malignancies and related diseases, including MS. Understanding the viral and host genetic basis of susceptibility to EBV-related disease may contribute to the development of preventive vaccines and immunotherapy treatments.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal