Over the last 2 decades, the focus of acute myeloid leukemia (AML) classification has gradually shifted from the microscope to miniaturized chips of increasingly sophisticated next-generation sequencing platforms. The French-American-British (FAB) classification for AML, based on morphological differentiation features and conceptualized in the 1970s, included 2 rare syndromes: FAB AML M6 (acute erythroid leukemia [AEL]) and FAB AML M7 (acute megakaryoblastic leukemia [AMKL]). According to the World Health Organization (WHO) 2001 classification, the diagnosis of AEL required the presence of at least 50% erythroid cells and ≥20% blasts in the non-erythroid compartment. The WHO 2008 revision partitioned AEL into an erythroid/myeloid (M6a) subtype (requiring ≥50% erythroid precursors and ≥20% myeloblasts within the total nucleated fraction) and a pure erythroid (M6b) subtype (requiring >80% erythroid precursors and ≥30% proerythroblasts). In the WHO 2016 update, the term AEL erythroid/myeloid subtype was eliminated and pure erythroid leukemia was retained. In the current WHO 2022 iteration, the term AEL has been reinstated and its association with biallelic TP53 defects, complex karyotype, therapy-related disease, and poor prognosis emphasized.1 AMKL is diagnosed by the presence of ≥20% marrow blasts, with >50% of the blasts megakaryoblastic or phenotypically positive for factor VIII, CD41, CD42, or CD61.2 AMKL has a favorable outcome in children with Down syndrome (DS), in contrast to poorer outcomes in non-DS children and adult patients. DS-related AMKL is etiologically associated with truncated GATA1, whereas in children lacking DS, the presence of chimeric RBM15::MKL-1 or CBFA2T3::GLIS2 is more prominent.3 Collectively, these diagnostic entities are exceptionally rare. AEL comprises <5% and AMKL comprises ∼1% of adult AML, with AMKL being more common in the pediatric population. Expected survival outcomes in adults are poor, ranging between 6 to 8 months and highlighting AEL and AMKL as orphan diseases with high unmet therapeutic need.2,4
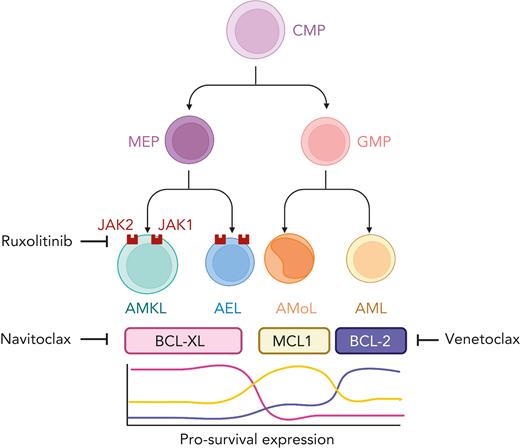
In this issue of Blood, Kuusanmäki et al5 cast a spotlight on the functional dependence of AEL and AMKL on pro-survival B-cell lymphoma-extra large (BCL-xL) and their cognate vulnerability to small molecule targeting by BCL-xL inhibitors. The authors combined drug profiling with single-cell transcriptomic approaches performed on both leukemic and normal hematopoietic populations and concluded that erythroid and megakaryocytic differentiation states are powerful determinants of pro-survival BCL-xL overexpression and dependency in AEL and AMKL (see figure). These findings extend the observations previously linking monocytic leukemia to MCL1 and chronic lymphocytic and differentiated myeloid leukemia to BCL-2 overexpression and synthetic vulnerability.6 The essentiality of erythroid and megakaryocytic cells to BCL-xL targeting was orthogonally supported by analysis of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) and RNAi-based genetic screens. Interestingly, in 3 xenograft models (2 AEL and 1 AMKL), each harboring pathogenic TP53 hotspot variants, the combination of A-1331852 and ruxolitinib, targeting BCL-xL and JAK1/2, respectively, synergistically suppressed systemic leukemia, suggesting that the activity of this combination could be TP53 independent; a relevant observation given the prevalence and poor risk conferred by TP53 variants in both AEL and AMKL.
Blasts with erythroid or megakaryocytic differentiation (AEL and AMKL, respectively) express high levels of BCL-xL and are uniquely vulnerable to BCL-xL inhibition. In contrast, BCL-2 and MCL1 expression are generaly increased in acute myeloid (AML) and monocytic (AMoL) leukemia, respectively. AEL and AMKL also demonstrate sensitivity to the JAK inhibitor, ruxolitinib, and the combined inhibition of BCL-xL and JAK leads to enhanced cell death. Figure created with BioRender.com.
Blasts with erythroid or megakaryocytic differentiation (AEL and AMKL, respectively) express high levels of BCL-xL and are uniquely vulnerable to BCL-xL inhibition. In contrast, BCL-2 and MCL1 expression are generaly increased in acute myeloid (AML) and monocytic (AMoL) leukemia, respectively. AEL and AMKL also demonstrate sensitivity to the JAK inhibitor, ruxolitinib, and the combined inhibition of BCL-xL and JAK leads to enhanced cell death. Figure created with BioRender.com.
The importance and clinical relevance of the current work by Kuusanmäki et al are sharpened by the dominant role of venetoclax as the treatment of choice for older patients considered unfit for intensive chemotherapy. The link between AEL and AMKL, pro-survival BCL-xL dependence, and TP53 dysfunction will likely limit the clinical effectiveness of venetoclax-azacitidine, providing a platform for BCL-xL inhibitor-based approaches to be explored in the clinic. Preclinical studies with the first generation BH3-mimetic ABT-737 have previously highlighted the importance of BCL-xL for platelet survival.7 Subsequently, first-in-human trials involving navitoclax (ABT-263) targeting BCL-2, BCL-xL, and BCL-W confirmed the occurrence of acute thrombocytopenia as an on-target consequence of BCL-xL targeted drugs in the clinic.8 Although it seems a formidable task to deploy a drug known to cause thrombocytopenia in patients with already compromised megakaryopoiesis, as likely to be the case in AEL and AMKL, current experience with BH3-mimetics in the setting of myelodysplastic syndromes and AML suggest that most patients achieve remission and recover hematopoietic function within just 1 to 2 cycles of therapy.9 Once marrow function is restored, on-target thrombocytopenia caused by BCL-xL antagonists may be more readily counterbalanced by a proportionate increase in platelet production by megakaryocytes.8 Reassuringly, navitoclax has been administered using a weekly dose ramp-up schedule in combination with ruxolitinib, with the regimen found to be feasible and clinically effective in patients with myelofibrosis.10 This suggests that novel regimens seeking to combine BCL-xL and JAK antagonists may be an achievable goal, even among patients with already compromised marrow function. We should anticipate, however, that lessons learned in the clinic regarding venetoclax resistance and emergence of subpopulations with reduced on-target binding affinity to BH3-mimetics, upregulation of off-target pro-survival partners (such as MCL1) and impaired function of proapoptotic TP53 or BAX/BAK will likely be a recurring theme.
Overall, the research findings by Kuusanmäki and et al reinforce the importance of cell of origin when considering BH3-mimetic therapies and provide a rationale for the clinical development of BCL-xL inhibitors, potentially in combination format, for patients with acute erythroid and acute megakaryoblastic leukemia. Ironically, this study also reinforces the need to retain the FAB nomenclature in AML classification to enable the diagnosis of AEL and AMKL to be made and affirms that the humble light microscope is not yet ready to be retired to the pages of diagnostic antiquity.
Conflict-of-interest disclosure: A.H.W. has served on advisory boards for Novartis, Astra Zeneca, Astellas, Janssen, Jazz, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, BMS, Shoreline, Macrogenics, Novartis, and Agios; receives research funding to the Institution from Novartis, AbbVie, Servier, Janssen, BMS, Syndax, Astex, AstraZeneca, and Amgen; serves on speaker’s bureaus for AbbVie, Novartis, BMS, Servier, and Astellas; and is an employee of the Walter and Eliza Hall Institute (WEHI). WEHI receives milestone and royalty payments related to the development of venetoclax. Current and past employees of WEHI may be eligible for financial benefits related to these payments. A.H.W. receives such a financial benefit. F.C.B. declares no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal