In this issue of Blood, Panelli et al1 identify a specific gene expression signature of a CD117+CD82+ cell subpopulation with enhanced leukemia-initiating properties, which is enriched in the minimal residual disease (MRD) of T-cell acute lymphoblastic leukemia (T-ALL) and in early T-cell progenitor acute lymphoblastic leukemia (ETP-ALL), a T-ALL subtype known for its poor prognosis. Interestingly, the authors uncover that this transcriptomic signature relies on a previously unknown noncanonical interaction of β-catenin with forkhead box-3 (FOXO3) transcription factor.
T-ALL is an aggressive hematological malignancy characterized by the acquisition of genomic abnormalities in T-cell progenitors. These genetic lesions induce common traits critical for the induction and/or maintenance of T-ALL, such as T-cell differentiation arrest, uncontrolled proliferation, and cell death alteration.2 Adults in particular with relapsed T-ALL or ETP-ALL have a poor prognosis, with bone marrow (BM) transplantation their main therapeutic option. A deep understanding of the cellular and molecular mechanisms involved in the leukemogenesis and in the relapse processes will surely point to new ways to improve patient care.
Many previous studies focused on intracellular/genetic events that could be targeted, such as oncogenic addiction and/or dependency to antiapoptotic proteins, for instance B-cell lymphoma–extra large (Bcl-xL), encoded by the BCL2-like 1 gene.3 As chimeric antigen receptor (CAR) T-cell–based therapies have shown a great efficacity in B-cell acute lymphoblastic leukemia (B-ALL), some groups have developed CAR T cells targeting T-cell surface markers, such as CD1a or CD7.4 This latter technology, albeit expensive, adds a novel therapeutic tool to the medical kit to treat T-ALL in relapsed patients.
In addition to intracellular oncogenic events, extracellular signals provided by specific cells in the microenvironments can impact blast cell growth and chemoresistance, thus representing another mechanistic layer to potentially exploit. Studying extracellular events requires that several different microenvironments have to be explored. T-ALL cells disseminate from the thymus, where the initial leukemic progression takes place, to many tissues, including the BM, central nervous system, and adipose tissue, via the peripheral blood. Depending on the microenvironment they invade, T-ALL cells may display different behaviors. They may be dependent on the CXCL12-CXCR4 pathway, which allows them to home and proliferate in BM niches (see figure).5 Hypoxia-inducible factor-1α stabilization, via low oxygen levels, supports Wnt/β-catenin activation that sustains T-ALL initiating activity,6 an observation supported by the fact that hypoxia, as is present in BM and adipose tissues, induces chemoresistance.7 T-ALL cells can also hijack dendritic cells that provide beneficial niche factors.8 Besides, adipocyte-rich BM can modify T-ALL/B-ALL metabolic features and consequently alter their chemosensitivity.9,10 Together, these studies provide compelling evidence of connections between T-ALL and its microenvironment, providing alternative pathways for therapeutic targeting. Deciphering molecular mechanisms that specifically lead to minimal residual disease/chemoresistance and ETP-ALL growth still need exploration.
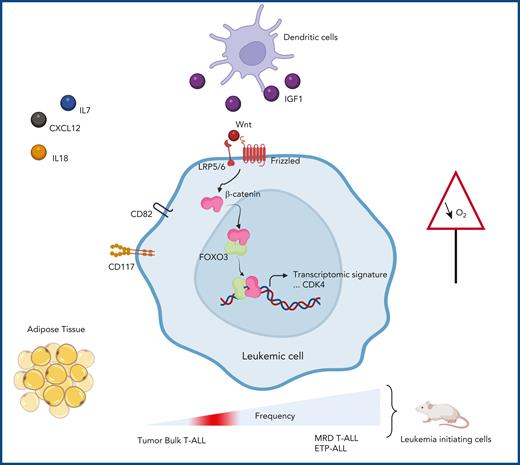
Factors in the microenvironment differentially impact the behavior of T-ALL cells. Some support leukemic cell expansion (IL18, IL7, CXCL12, IGF1 provided by dendritic cells), whereas others protect them against chemotherapy (adipose tissue and hypoxia). The work of Panelli et al reveals a noncanonical interaction between β-catenin and FOXO3. This interaction induces a gene expression signature typically found in a chemoresistant cell population expressing CD82 and CD117/receptor tyrosine kinase (KIT). These cells are found enriched in early T-ALL and MRD of T-ALL and are endowed with high capacity of leukemia initiation in immunodeficient mice. CDK4, cyclin-dependent kinase 4; Wnt, wingless-related integration site.
Factors in the microenvironment differentially impact the behavior of T-ALL cells. Some support leukemic cell expansion (IL18, IL7, CXCL12, IGF1 provided by dendritic cells), whereas others protect them against chemotherapy (adipose tissue and hypoxia). The work of Panelli et al reveals a noncanonical interaction between β-catenin and FOXO3. This interaction induces a gene expression signature typically found in a chemoresistant cell population expressing CD82 and CD117/receptor tyrosine kinase (KIT). These cells are found enriched in early T-ALL and MRD of T-ALL and are endowed with high capacity of leukemia initiation in immunodeficient mice. CDK4, cyclin-dependent kinase 4; Wnt, wingless-related integration site.
In their article, Panelli and colleagues uncover a novel noncanonical interaction between activated β-catenin (activation due to extracellular Wnt) and FOXO3. This noncanonical β-catenin/FOXO3 relation activates specific genes, including CDK4 (see figure), known to regulate cell cycling, thus promoting leukemia expansion. Interestingly, the β-catenin/FOXO3–related expression signature helps define leukemic cells endowed with an enhanced ability to initiate leukemia in immunodeficient mice. Moreover, this cell population, which is highly enriched in T-ALL MRD and in ETP-ALL, expresses 2 cell surface markers: CD82, a member of the tetraspanin family; and CD117/KIT, the stem cell factor receptor. Panelli and colleagues provide additional arguments on how the microenvironment (here through Wnt ligands) impacts leukemic growth and chemoresistance by uncovering a cell subpopulation with a unique transcriptomic signature. The identification of these new functional roles for β-catenin and FOXO3 by Giambra et al6 shows common vulnerabilities of MRD and ETP-ALL, which may be targeted.
Emerging strategies seek to drastically improve the patients’ care, especially when chemotherapy fails. However, some of these therapies are costly, and the strong plasticity of leukemic cells unfortunately participates in leukemic evasion and thus leads to relapse. The work by Panelli et al, by connecting cell-autonomous and non–cell-autonomous signals, may be instrumental in improving ETP/T-ALL cure rates in the coming years.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal