Abstract
Introduction Early treatment initiation for patients with high-risk CLL has not been shown to be associated with clinical benefit in studies utilizing rituximab and rituximab in combination with alemtuzumab. Ofatumumab is a fully human monoclonal antibody targeting CD20. It is well-tolerated and has improved target binding and cytotoxicity compared to rituximab. We therefore sought to evaluate the efficacy of ofatumumab as early treatment in patients with high-risk CLL.
Methods This was a single-arm, open label, phase II trial for treatment-naïve, watch-and-wait patients with high-risk for progression to need treatment. Based on currently available models, high-risk CLL was defined as presence of one or more of the following: Rai stage 2 disease or Rai stage 0-1 with disease related fatigue, β2M ≥ 3mg/L, ALC ≥ 25K/µL, unmutated IGHV, ZAP70-positive, CD38-positive, or FISH with del(11q) or del(17p). Patients who met 2008 iwCLL criteria for treatment were excluded. The primary objective was overall response rate (ORR) with secondary objectives of progression-free survival (PFS), time-to-next treatment (TTNT), and overall survival (OS). Treatment consisted of a total of 8 weekly doses of ofatumumab. The first weekly dose was 300mg to reduce infusion reactions, subsequently ofatumumab 1000mg was then administered weekly for 7 weeks.
Results Between 04/2011 and 06/2015, 44 pts were enrolled in the study and initiated treatment. The median age at study entry was 60 years with a median time from diagnosis to study entry of 15.3 mo. The median ALC, HGB and PLT counts were 20.1K/µL, 14mg/dL, and 166K/µL, respectively. The median LDH was 481U/L (range 345 - 855). When characterized based on FISH alterations, 19(43%) pts had del(13q), 7(16%) pts had trisomy 12, 8(18%) pts had del(11q), 3(7%) pts had del(17p), and 7(16%) pts had no abnormalities. When characterized based on other high-risk features: 24(55%) pts had unmutated IGHV, 21(48%) pts were ZAP70(+), 15(34%) pts were CD38(+), 6(14%) had elevated β2M, and 21(48%) had an ALC over 25K/µL.
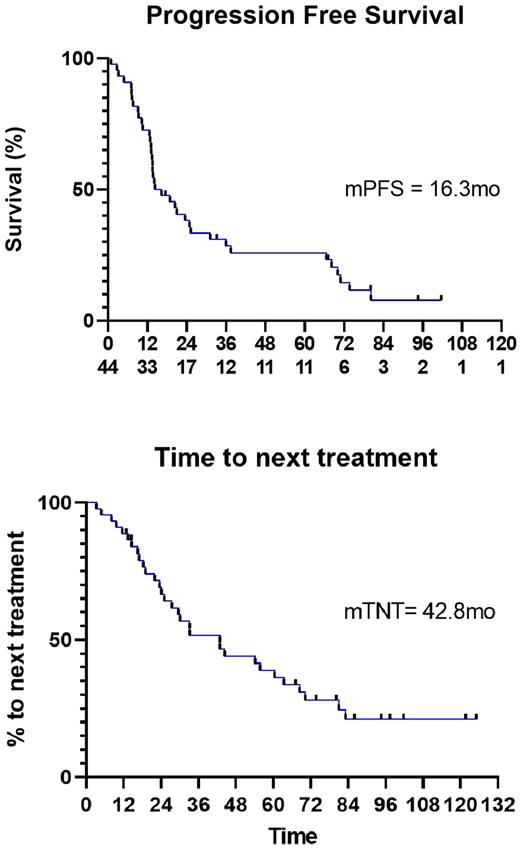
The ORR to ofatumumab (complete + partial remission) was 63% with 3(7%) pts attaining undetectable MRD status at 10-4 sensitivity. At a median follow up of 8 years, the median PFS, TTNT, and overall survival (OS) were 16.3 mo, 42.8 mo, and not reached. Thirty (68%) pts went on to receive further treatment (19 pts on BTK based regimens, 2 pts received venetoclax monotherapy, 5 pts received combined venetoclax and ibrutinib, 3 pts received chemoimmunotherapy, and 1 pt received obinutuzumab monotherapy).
This regimen of early treatment with ofatumumab was well tolerated. The most common adverse reaction was infusion reactions occurring in 16 pts (36%) with all reactions being amendable to antihistamine and/or steroid treatment. In all of these pts the infusion was restarted. One pt had to discontinue ofatumumab treatment after the second infusion due to autoimmune hepatitis.
Conclusion In a population of patients with CLL at high-risk for proceeding to treatment, early treatment with ofatumumab resulted in a PFS and TTNT of 16.3 mo and 42.8 mo, respectively. The PFS was similar to that of similarly designed trials with rituximab monotherapy and rituximab in combination with alemtuzumab, respectively. Further internal matched analysis is being conducted to compare time to second treatment.
Disclosures
Ferrajoli:Beigene: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Jain:Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Fate Therapeutics: Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Cellectis: Honoraria, Research Funding; MEI Pharma: Honoraria; Dialectic Therapeutics: Research Funding; CareDx: Honoraria; Newave: Research Funding; Loxo Oncology: Research Funding; Takeda: Research Funding; Incyte Corporation: Research Funding; Medisix: Research Funding; Ipsen: Honoraria; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; TransThera Sciences: Research Funding; TG Therapeutics: Honoraria; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novalgen: Research Funding; Aprea Therapeutics: Research Funding; Pfizer: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; ADC Therapeutics: Research Funding; Beigene: Honoraria; Cellectis: Honoraria, Research Funding; Servier Pharmaceuticals LLC: Research Funding; Mingsight: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. DiNardo:Servier: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Forma: Research Funding; Foghorn: Honoraria, Research Funding; Astex: Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Gilead: Honoraria; Bluebird Bio: Honoraria; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees; Cleave: Research Funding; Novartis: Honoraria; ImmuneOnc: Honoraria, Research Funding; LOXO: Research Funding; Jazz: Honoraria. Konopleva:Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; Forty-Seven; F. Hoffman LaRoche: Honoraria; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding. Kadia:Ascentage: Research Funding; Novartis: Consultancy; Glycomimetics: Research Funding; cyclacel: Research Funding; Amgen: Research Funding; PinotBio: Consultancy; AstraZeneca: Research Funding; Delta-Fly: Research Funding; JAZZ: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy; Pfizer: Research Funding; Genfleet: Research Funding; cellenkos: Research Funding; Regeneron: Research Funding; Astellas: Research Funding; Astex: Honoraria; Iterion: Research Funding; BMS: Consultancy, Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. O'Brien:Acerta, Alliance, Beigene Ltd, Caribou Biosciences Inc, Gilead, Kite, Loxo Oncology, Mustang, Nurix Therapeutics Inc, Pfizer, Pharmacyclics, Regeneron, Sunesis, and TG Therapeutics.: Research Funding; AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, Bristol Myers Squibb, Celgene, DynaMed, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma Inc, Merck, NOVA Resea: Consultancy. Burger:Pharmacyclics LLC: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; BeiGene: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AstraZeneca: Research Funding; Novartis: Honoraria, Other: Travel, Accommodations, Expenses. Thompson:AbbVie, Pharmacyclics, Adaptive Biotechnologies, Genentech: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech: Consultancy; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech, Amgen: Honoraria. Wierda:Bristol Meyers Squibb (Juno and Celgene): Research Funding; Juno: Research Funding; Karyopharm: Research Funding; Kite, a Gilead Company: Research Funding; Cyclacel: Research Funding; Genentech: Research Funding; Gilead Sciences: Research Funding; GSK/Novartis: Research Funding; Janssen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Sanofi: Consultancy; AstraZeneca/Acerta Pharma. Inc.: Research Funding; Genzyme: Consultancy; Xencor: Research Funding; Sunesis: Research Funding; Pharmacyclics LLC: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal