Abstract
Background: In acute myeloid leukemia (AML), clonal hematopoiesis (CH)-associated mutations usually appear early in leukemogenesis, and often persist in complete remission following chemotherapy. The presence of residual CH at the time of a consolidating allogeneic hematopoietic stem cell transplantation (HSCT) has no negative impact on outcomes of AML patients (pts). Here, we analyzed the prognostic impact of measurable residual CH (MRCH) early after HSCT.
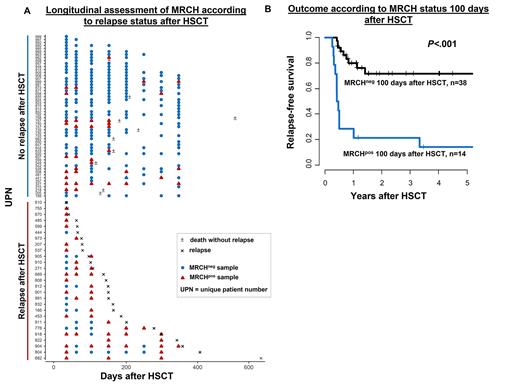
Results: In remission before HSCT 83% of pts remained MRCHpos, which did not associate with a higher cumulative incidence of relapse (CIR, P=.19) or shorter relapse-free survival (RFS, P=.08, with a trend for higher CIR in patients without persisting MRCH). Fifty-one (35%) pts relapsed after HSCT. In 29 of these pts material at relapse was available and showed that the CH mutation detected at diagnosis was also present in all but one relapse samples (median VAF at relapse of 11.8 [range 0.2-68.3]%). Of those, 25 pts had at least 1 post-HSCT sample available prior to relapse. In 22/25 pts, impeding relapse was preceded by detectable MRCH (first positive sample with a median VAF of 0.25 [range 0.06-12.3]) at a median of 55 days prior to morphologic relapse. In two non-converting (conversion from MRCHneg to MRCHpos) patients who relapsed, both with SRSF2 mutations, the last samples 132 and 35 days prior to relapse were MRCHneg. One non-converting patient suffered from an extramedullary relapse without bone marrow involvement. A total of 19% of pts died without relapse. Of the pts alive in remission, the majority remained MRCHneg (Figure 1A).
At all evaluated timepoints post-HSCT, we observed a significantly higher CIR and shorter RFS for AML pts in morphologic remission who had detectable MRCH: at day 28 after HSCT (P=.01, and P<.001, respectively), day 100 after HSCT (P<.001, and P<.001, respectively, Figure 1B), day 180 after HSCT (P=.003, and P=.002, respectively), and day 360 after HSCT (P=.001, and P=.04, respectively). While the risk of relapse remained high in MRCHpos pts irrespective of the timepoint of assessment during post-HSCT follow-up (46-66% after 3 years), the 3-year risk of relapse in MRCHneg pts continuously decreased with time after HSCT (28% at day +28, 13% at day +100, 5% at day +180, 0% at day +360 in MRCHneg pts, respectively).
Conclusions: In contrast to the absent prognostic relevance of persisting MRCH in remission before HSCT, the presence of detectable MRCH after HSCT was linked to a higher CIR and shorter RFS at every evaluated timepoint early after HSCT. The risk of relapse in MRCHneg pts continuously decreased within the first year after HSCT: the longer pts remained MRCHneg the less likely relapse occurred. Our data suggests MRCH as a feasible marker for residual disease assessment in AML pts in morphologic remission after allogeneic HSCT.
Disclosures
Merz:Janssen: Honoraria; BMS Celgene: Honoraria. Herling:Novartis: Honoraria. Metzeler:Curis Inc: Research Funding; Celgene: Consultancy; Abbvie: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Pfizer: Consultancy. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Schwind:Novartis: Honoraria. Jentzsch:Pfizer: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal