Abstract
Myeloproliferative neoplasms (MPNs) are clonal hematological diseases driven by somatic mutations acquired in hematopoietic stem cells (HSCs) and characterized by the deviant, unregulated proliferation of one or more myeloid lineages. Chronic MPN can present as polycythemia vera (PV; excess erythrocytes), essential thrombocythemia (ET; excess platelets), or myelofibrosis (MF; bone marrow fibrosis). More than 300,000 patients are currently living with an MPN diagnosis in the United States.
The JAK2V617F variant is an acquired somatic mutation recurrently found in MPN patients and is used as a diagnostic marker along with increased hematocrit, increased hemoglobin, and adverse thrombotic events. In MPN patients, the frequency of JAK2V617F mutant cells varies widely but is known to induce an MPN phenotype with both high (>50%) and very low (<5%) variant allelic frequency (VAF). The reason for this is currently unknown. Most JAK2-mutant animal models model a VAF which is much higher than the lower end of detectable VAFs found in MPN patients. This results in a very limited understanding of what the minimal mutant cell burden required for disease manifestation is. The understanding of genotype/phenotype relationships in patients with low JAK2V617F VAF chronic MPN is paramount to our understanding of this disease and its hematological progression.
To this end, we have designed a clinically relevant, humanized mouse model wherein we employ CRISPR and homology directed repair (HDR) via a single stranded oligo donor nucleotide sequence (ssODN) to knock-in the JAK2V617Fmutation at the endogenous locus of human cells at low VAF. In addition, a synonymous JAK2V617V mutation was introduced via CRISPR/ssODN in parallel cells to serve as a control tracked by VAF alongside the VAF of JAK2V617F in their corresponding cohorts. The respective ssODNs were nucleofected into either umbilical cord blood (UCB) derived CD34+ cells or healthy adult bone marrow (BM) derived CD34+ cells to investigate the possibility that developmental age is a variable in low JAK2V617F VAF inducing a clinical MPN phenotype. After nucleofection, 20,000 CD34+ cells were intra-tibially transplanted into sub-lethally irradiated (250 rads) NSGS mice. The low cell input was utilized to limit engraftment and initial disease burden of mutant cells. Effect was measured by mutant allele burden by droplet-digital PCR (ddPCR) analysis, flow cytometry at selected timepoints to track human cell engraftment, spleen weight, and complete blood count (CBC) analysis including hematocrit, hemoglobin, white blood cells, and platelets.
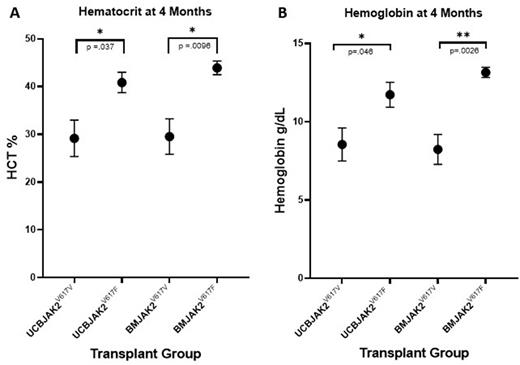
At 4 months post-transplant, peripheral blood hCD45+ engraftment across all cohorts averaged less than 3%. Average VAF of circulating hCD45+ cells increased in both JAK2V617F cohorts 5 months post-transplant compared to day 0 post nucleofection. Notably, overall JAK2V617F VAF in each sample remained <5% when adjusted to include both human and mouse derived peripheral blood cells. Remarkably, both hematocrit (p= .037 in UCB cohorts and p=.0096 in BM cohorts; Figure 1A) and hemoglobin (p=.046 in UCB cohorts and p=.0026 in BM cohorts; Figure 1B) were significantly elevated in both JAK2V617F cohorts compared to their respective JAK2V617V controls, while white blood cell and platelet counts remained unchanged within normal ranges. In addition, spleen weights were significantly increased in both JAK2V617F cohorts compared to their respective controls.
These data demonstrate a successful clinical model of MPN which induces a classical MPN phenotype where a low VAF of JAK2V617F corresponds with a significant increase of hematocrit, hemoglobin, and spleen weight in a humanized mouse model compared to relevant controls. In addition, we demonstrate that while engraftment due to low hCD34+ cell input remains low after 4 months, the VAF of JAK2V617F within the hCD45+ sub- population increases. This successfully enables the overall VAF contributing to the MPN phenotype to remain at clinically relevant lower levels compared to other models with much higher VAF contributions. We believe this model to be of high clinical relevance into the investigation of how low JAK2V617F burden relates to disease development and progression in MPN patients.
Disclosures
Challen:Incyte: Consultancy, Other: Sponsored Research agreements.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal