Abstract
Introduction: Secondary CNS lymphoma (SCNSL) is a challenging clinical scenario observed in 2-5% of non-Hodgkin lymphoma patients, without well-defined standard of care. We sought to understand the indications and outcomes of patients with SCNSL referred for radiotherapy (RT) at a tertiary cancer center.
Methods: We analyzed 93 consecutive patients with SCNSL of aggressive B cell histology who received brain RT at Memorial Sloan Kettering Cancer Center between 1999-2021. Overall survival (OS) was estimated using the Kaplan-Meier method. Time to intracranial progression was estimated for patients with brain imaging at least 30 days after treatment start using competing risk analysis with death as a competing risk. Outcomes were considered from the start of RT. Patients were further analyzed by treatment intent and by the presence of leptomeningeal disease (LMD), defined radiographically or by cerebrospinal fluid analysis.
Results: Median age was 63 (interquartile range [IQR]: 51-70). 9% of patients had CNS involvement at initial diagnosis. Histologies included diffuse large B cell (76%), mantle cell (7%), post-transplant lymphoproliferative disorder (7%), Burkitt (5%), Richter's transformed CLL (4%), lymphoblastic lymphoma (1%). 55% of patients received R-CHOP and 12% received R-EPOCH as initial therapy, and 25% received CNS prophylaxis. 21 (23%) patients received RT as their initial SCNSL treatment. Of the 72 (77%) patients who received CNS-directed systemic therapy for SCNSL prior to brain RT, 88% received a methotrexate (MTX)-based regimen. Median time interval between MTX and RT was 32 days (IQR: 15 - 82). 86 (92%) of patients received whole brain (WBRT) and 7 (8%) received partial brain RT. Median RT dose was 30 Gy (IQR: 23.4-30). 3 patients received craniospinal irradiation (CSI).
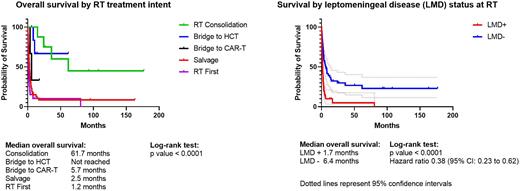
Median OS for the entire cohort was 2.9 months. Median OS differed by RT treatment intent as follows: consolidation after complete response to systemic therapy (61.7 months, n=8), RT bridging to hematopoietic cell transplantation (HCT) (not reached, n=7), RT bridging to CAR-T (5.7 months, n =3), RT salvage after progression or intolerance of systemic therapy (2.5 months, n = 54), RT as initial SCNSL treatment typically in an emergency or palliative setting (1.2 months, n=21) (p < 0.0001). Across the full cohort, median OS was longer for patients without evidence of LMD at the time of RT (6.3 vs. 1.7 months, p < 0.0001). Among patients with LMD at SCNSL diagnosis, those who cleared LMD by the time of RT had longer median OS than those with persistent LMD (8.3 vs. 2.2 months, p = 0.014). The estimated 3-year risk of intracranial failure after salvage RT was 35 ± 11% for patients without LMD and was not evaluable for patient with LMD at salvage as all patients' treatment failed or the patients died by 4 months.
Conclusion: SCNSL remains a clinical challenge and patients referred for brain RT have overall poor prognosis. However, the subset of patients who receive RT as consolidation or bridge to HCT can achieve long term survival. The presence of LMD at RT is prognostic for inferior survival. For SCNSL patients without LMD, intracranial disease control after salvage RT is possible. Alternate treatment strategies including broader consideration of CSI for persistent LMD are warranted. Further identification of clinical, anatomic, and molecular subtypes of SCNSL that impact treatment response will help to clarify optimal treatment strategies.
Disclosures
Scordo:Angiocrine Bioscience, Inc.: Consultancy, Research Funding; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); McKinsey & Company: Consultancy; i3Health (CME): Honoraria; Medscape, LCC (CME): Honoraria. Grommes:Ampressa Therapeutics, Inc: Other: provision of services; BTG International: Other: provision of services; Kite Pharmaceuticals: Other: provision of services; Ono Pharma: Other: provision of services; Scripps Conference Services & CME: Other: provision of services. Imber:GT Medical Technologies: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal