Abstract
Introduction UKALL 2011 randomised children and young adults (up to 25) with Acute Lymphoblastic Leukaemia (ALL) or Lymphoblastic Lymphoma (LBL). The aims were to reduce induction toxicity (R1: Short [14 days] vs Standard [28 days] dexamethasone), CNS relapse risk (R2IM: HD MTX (HDM) vs standard interim maintenance (SIM)) and maintenance morbidity (R2pulses: Vincristine (VCR)/dexamethasone pulses or not). R1 results were presented at ASH 2017 and did not show a reduction in toxicity with short dexamethasone. Results of R2IM and R2pulses are reported here.
Patients and Methods Patients were stratified by NCI risk, cytogenetics and end of induction MRD to receive Regimens A (NCI standard risk, MRD low risk), B (NCI high risk, MRD low risk) or C (Cytogenetic poor risk or MRD intermediate risk regardless of NCI risk). MRD high risk (end of consolidation MRD >0.5%, n=14) were not eligible for randomisation and received off protocol therapy. R2 was a factorial randomisation stratified by factors including NCI and MRD risk groups and resulted in 4 arms: HDM with pulses, HDM without pulses, standard interim maintenance (SIM) with pulses (standard of care) and SIM without pulses. SIM was for 2 months with oral mercaptopurine/MTX, monthly pulses and single IT MTX in Regimens A and B, and 5 doses of escalating IV MTX (Capizzi) + VCR + 2 doses of Pegylated asparaginase in Regimen C. HDM was given at a dose of 5g/m2 x 4 doses 2 weeks apart, low dose 6-MP and 2 doses of Pegylated asparaginase (Regimen C only). The primary endpoint for R2IM was the CNS relapse rate (CNSR) and for R2pulses was the bone marrow relapse rate (BMR) in ALL only; non-inferiority, 5% margin at 5 years. Event Free Survival (EFS) was considered a primary endpoint for both.
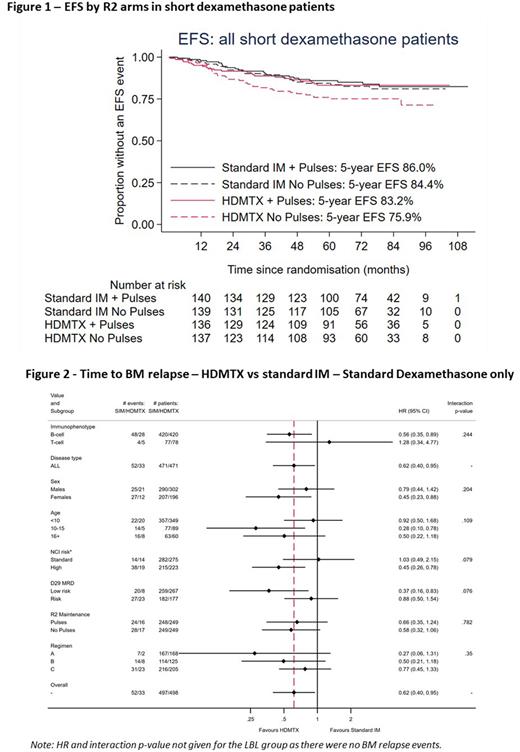
Results Of the 2750 patients registered on trial between April 2012 and December 2018, 1902 were randomised to R1 (closed April 2017) and 1570 to R2. Median age 5 years (IQR: 3-11), 83% B-ALL, 12% T-ALL, 1% B-LBL and 4% T-ABL, 43% NCI high risk. Median follow-up for R1 is 76 months and R1 or R2 is 72 months. Overall, we found no difference in CNSR (HR: 0.99 (0.65-1.51), p=0.97, 5-year rates: 5.6% and 5.6%), or any other endpoints for R2IM. However, there was an interaction between the R1 and R2IM randomisations (p=0.006 for EFS) with inferior outcomes for patients treated with short dexamethasone followed by HDM, particularly in those who did not receive pulses (Figure 1). Limiting analyses to patients treated with standard dexamethasone (N=995, including those that received it after closure of R1) there was a significant reduction in BMR rate with HDM (HR 0.62 (95% CI: 0.40 - 0.95) p = 0.029) and a trend for improvements in EFS and OS (HRs: 0.75 (95%CI: 0.53 - 1.04), p=0.087 and 0.61 (0.37 - 1.03), p=0.067) but no difference in CNSR. In subgroup analyses there were no significant interactions, but the effect appeared stronger for B-lineage, NCI high risk and MRD low risk patients (Figure 2). Overall, the BMR for R2pulses was non-inferior (+2.1% increase BMR at 5 years for ALL patients (95% CI: -1.4% to 4.7%), HR 1.22 (95%CI: 0.89 - 1.67). Considering all four R2 treatment arms in standard dexamethasone only, standard IM without pulses has a lower EFS, whilst there is no appreciable benefit of pulses in HDM treated patients; 5-year EFS difference: -2.8% (95% CI: -7.4% to 4.4%) and BMR (ALL): +0.4% (-6.7% to 4.3%).
Conclusions HDM does not improve CNSR within a UKALL treatment backbone, but pre-specified analyses suggest it may improve BMR for some sub-groups of B-lineage patients when given following standard dexamethasone induction. Although the 'no pulses' arm was non-inferior overall for BMR, further sub-group and toxicity analyses are to be performed and will presented at the meeting.
Acknowledgements Children with Cancer, Blood Cancer (grant ref 09042) and Cancer Research UK funded the trial.
Disclosures
Kirkwood:Kite: Consultancy, Honoraria. Rowntree:Pfizer: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; KITE pharma: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal