Abstract

Background: Ponatinib and blinatumomab both have single-agent activity in Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). The combination of these two active agents could be an effective chemotherapy-free strategy for patients (pts) with Ph+ ALL and may reduce the need for allogeneic stem cell transplantation (SCT) in first remission.
Methods: In this phase II study, pts ≥18 years of age with newly diagnosed Ph+ ALL were eligible. Pts who had received 1-2 previous cycles of chemotherapy, with or without a BCR::ABL1 TKI, could still be enrolled. Pts were required to have a performance status of ≤2, total bilirubin ≤2x the upper limit of normal (ULN), and ALT and AST ≤3x the ULN. Pts with uncontrolled cardiovascular disease or clinically significant central nervous system (CNS) comorbidities (except for CNS leukemia) were excluded. Pts received up to 5 cycles of blinatumomab as a continuous infusion at standard doses. Ponatinib 30mg daily was started during cycle 1 and was decreased to 15mg daily once CMR was achieved. After 5 cycles of blinatumomab, ponatinib was continued for at least 5 years. Twelve doses of prophylactic IT chemotherapy with alternating cytarabine and methotrexate were administered. The primary endpoint was the complete molecular response (CMR) rate. Secondary endpoints included response rates, safety measures, event-free survival (EFS) and overall survival (OS).
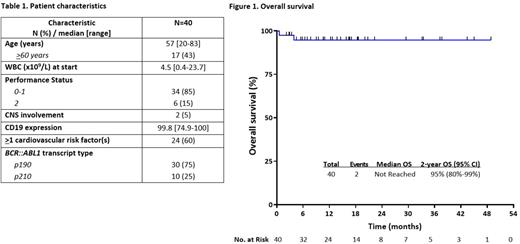
Results: Between 6/2018 to 5/2022, 40 pts with newly diagnosed Ph+ ALL were enrolled. Baseline characteristics are shown in Table 1. The median age was 57 years (range, 20-83 years); 30 pts (75%) had the p190 fusion protein. The median CD19 expression was 99.8% (range, 74.9-100%). 12 pts were in CR at enrollment (2 of whom were in CMR and were evaluable only for relapse and survival outcomes).
Among the 28 pts who were evaluable for hematologic response, 27 (96%) achieved CR or CRi, with CR achieved in 26 pts (93%). One pt died on day 18 from intracranial hemorrhage in the setting of cytoreductive chemotherapy administered prior to enrollment. All hematologic responses occurred after 1 cycle. Among the 38 pts who were evaluable for CMR, 26 pts (68%) achieved CMR after cycle 1, and 33 pts (87%) achieved CMR at any time. Molecular responses occurred rapidly, and after only 2 weeks of therapy, 15 of 20 tested pts (75%) had achieved CMR in the peripheral blood. Twenty-two of 25 tested pts (88%) also became MRD-negative by an NGS assay with sensitivity of 1x10-6 (clonoSEQ assay from Adaptive Biotechnologies Co.). Three of these pts who were MRD-negative by NGS had detectable low-level BCR::ABL1 transcripts by PCR at the same time (ranging from 0.01% to 0.05%). No patients were negative for BCR::ABL1 by PCR but positive by NGS.
The median number of cycles received was 5 (range, 1-5). Among 39 patients who received at least 1 complete cycle of ponatinib-blinatumomab, one pt died in CR due to hypovolemic shock following cardiac catheterization, and the other 38 pts are in ongoing hematologic remission. Only 1 pt underwent SCT in first remission; this pt was transplanted due to persistently detectable BCR::ABL1 transcripts of 0.01%-0.05%.
The median follow-up is 15 months (range, 2-49 months). The estimated 2-year EFS and OS are both 95% (95% confidence interval, 80%-99%) (Figure 1). No relapses and no leukemia-related deaths have been observed. Among the 37 pts in ongoing remission without SCT, the median duration of response is 14 months (range, 1-44 months).
The treatment was well-tolerated, and most toxicities were grade 1-2 and consistent with the known toxicities of the two agents. There were no grade 4-5 related adverse events. Ponatinib was discontinued in 2 patients due to possibly related adverse events (CVA and coronary stenosis in 1 pt each).
Conclusion: Simultaneous ponatinib and blinatumomab is safe and effective in pts with newly diagnosed Ph+ ALL. This chemotherapy-free regimen results in high rates of CMR and NGS MRD negativity. Encouraging survival has been observed without the need for SCT.
Disclosures
Short:Novartis: Consultancy; Stemline Therapeutics: Research Funding; Pfizer: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy; Astellas: Research Funding. Kantarjian:Daiichi-Sankyo: Consultancy, Research Funding; NOVA Research: Honoraria; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria. Jain:Fate Therapeutics: Research Funding; Medisix: Research Funding; Aprea Therapeutics: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; TG Therapeutics: Honoraria; Loxo Oncology: Research Funding; ADC Therapeutics: Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pfizer: Research Funding; Ipsen: Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Cellectis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; MEI Pharma: Honoraria; Novalgen: Research Funding; Beigene: Honoraria; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; TransThera Sciences: Research Funding; Cellectis: Honoraria, Research Funding; Incyte Corporation: Research Funding; Dialectic Therapeutics: Research Funding; Newave: Research Funding; Servier Pharmaceuticals LLC: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Mingsight: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Takeda: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; CareDx: Honoraria. Kadia:Regeneron: Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy; Genfleet: Research Funding; Astellas: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding; cyclacel: Research Funding; PinotBio: Consultancy; Delta-Fly: Research Funding; Astex: Honoraria; Glycomimetics: Research Funding; Iterion: Research Funding; cellenkos: Research Funding; Ascentage: Research Funding; Pfizer: Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding; Agios: Consultancy; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Alvarado:Sun Pharma: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Astex Pharmaceuticals: Research Funding; BerGenBio: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding. Garcia-Manero:BMS: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; AbbVie: Honoraria, Research Funding; Gilead Sciences: Research Funding; Acceleron Pharma: Consultancy; Curis: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Issa:Novartis, Kura Oncology: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Novartis: Research Funding. Konopleva:Ascentage: Other: grant support, Research Funding; Ablynx: Other: Grant support, Research Funding; Calithera: Other: Grant Support, Research Funding; Agios: Other: grant support, Research Funding; Cellectis: Consultancy, Other: Grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Rafael Pharmaceutical: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Novartis: Patents & Royalties, Research Funding; Eli Lilly: Consultancy, Patents & Royalties, Research Funding; Reata Pharmaceuticals: Current equity holder in private company, Patents & Royalties; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kisoji: Consultancy, Honoraria; Forty-Seven: Consultancy, Honoraria, Other: Grant support; Amgen: Consultancy; F. Hoffman La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grant support, Research Funding; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Other: grant support, Research Funding; AbbVie: Consultancy, Other: grant support, Research Funding. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Novartis: Consultancy; Biomea Fusion, Inc.: Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding. Jabbour:Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal