Abstract
Resistance to ibrutinib treatment is an increasing problem for patients with chronic lymphocytic leukemia (CLL) as it leads to dismal clinical outcomes. Acquired mutations in the therapy target BTK or its downstream mediator PLCG2 explain only partly the non-responsiveness to this treatment, as merely a subset of patients harbor such mutations, or if so, only in a fraction of the malignant B cells. Besides genetic aberrations, molecular changes both CLL cell-intrinsic and -extrinsic have been associated with ibrutinib resistance. But so far these alterations have not led to the development of novel therapy approaches for patients who are non-responsive to ibrutinib treatment.
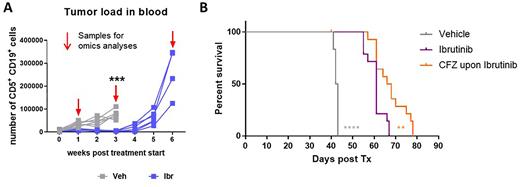
We have generated a mouse model of ibrutinib resistance by treating mice upon adoptive transfer of Eµ-TCL1 leukemia (TCL1 CLL) continuously with ibrutinib. For a period of several weeks, the leukemic mice respond very well to the treatment, but similarly as observed in patients, relapse under therapy occurs with an aggressive outgrowth of the malignant cells (Figure A), which are non-responsive to ibrutinib upon retransplantation.
By whole exome sequencing (WES) of TCL1 CLL cells before treatment and after relapse we did not detect any mutations in Btk or downstream signaling molecules in the resistant cells. In addition, the identified mutations were of low allele frequency and no recurrent mutations were detectable in the relapse samples that could explain the non-responsiveness to ibrutinib.
To analyze molecular adaptations that drive resistance to ibrutinib, we performed transcriptome and proteome analyses of TCL1 CLL cells from mice treated with ibrutinib or vehicle at an early responsive time point, and a late time point, when mice got refractory to treatment with ibrutinib (see arrows in Figure A). Comparative analyses of these complex data sets suggested alterations in the proteasome activity as a driver of ibrutinib resistance. By quantifying total ubiquitination of proteins, we further confirmed reduced levels of K48-ubiquitination which suggest an increased proteasomal activity in ibrutinib-resistant tumors.
Based on these results, we performed preclinical treatment studies with the proteasome inhibitor carfilzomib, which more efficiently controlled TCL1 CLL in ibrutinib-resistant than in ibrutinib-sensitive tumors. Most interestingly, when carfilzomib was added as salvage therapy at the time of ibrutinib-resistance development in mice, we observed a clear survival benefit compared to mice who continued on ibrutinib monotherapy (Figure B).
To verify the relevance of these findings for patients with ibrutinib-resistant CLL, we acquired proteome data of CLL cells from three sequential time points in 3 patients: (1) before start of ibrutinib treatment, (2) during ibrutinib responsiveness (within the first 6 months of treatment), (3) upon ibrutinib refractoriness. This allowed us to identify common dynamic clusters of deregulated proteins that we are currently exploring concerning their role in resistance development. In addition, we treated CLL cells from ibrutinib-resistant patients cultured under lymph node-mimicking conditions ex vivo with several proteasome inhibitors and showed effective responses of these cells comparable to CLL samples of ibrutinib-sensitive patients.
Altogether, our omics data approach identified proteasome inhibitors as attractive novel treatment options for CLL patients that do not respond to ibrutinib. Current investigations of proteome data acquired from TCL1 CLL samples before and during carfilzomib treatment aim at identifying the molecular targets of the proteasome with relevance for ibrutinib resistance.
Figure:A) Treatment response in mice with TCL1 CLL to ibrutinib (Ibr) and vehicle (Veh) measured as the number of CD5+ CD19+ CLL cells in blood over time. B) Survival curves of mice with TCL1 CLL treated either with vehicle or ibrutinib as monotherapy, or a combination starting with 2 weeks of ibrutinib treatment, followed by the addition of carfilzomib (CFZ).
Disclosures
Skånland:AstraZeneca: Honoraria; BeiGene: Research Funding; TG Therapeutics: Research Funding; AbbVie: Honoraria. Tausch:BeiGene: Honoraria; Janssen-Cilag: Honoraria; AstraZeneca: Honoraria, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau. Stilgenbauer:Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVIe: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Infinity: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sunesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Veristem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Seiffert:Bayer AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal