Abstract
Background: Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for patients with acute leukemia. Despite this, studies have shown that only a minority of patients that may benefit from transplant ultimately proceed to HCT (Estey, Blood, 2007). Historical barriers to HCT include the lack of a suitable donor; however, this should no longer be a major limitation given the availability of alternative graft sources. Furthermore, prior studies of HCT barriers are often limited to patients who only achieve a complete remission to induction therapy. We sought to prospectively determine barriers to HCT in all patients recommended for transplantation given the widespread availability of alternative donors.
Methods: Adult patients with acute leukemia were enrolled at the time of initial induction or re-induction therapy at Memorial Sloan Kettering Cancer Center (MSK). Initial transplant and donor recommendations for each patient were made by early consensus review between day 15-21 of (re-)induction. The primary objective was to identify the rate of HCT in patients for whom HCT was initially recommended, and to identify reasons why patients deemed appropriate and eligible for HCT did not subsequently proceed to transplant. Time to HCT was descriptively summarized, and overall survival (OS) was estimated via Kaplan-Meier, comparing the newly diagnosed patients who did vs. did not proceed to HCT by 6 months post-diagnosis. The secondary objective was to determine the concordance between the initially recommended and subsequently utilized stem cell donor type.
Results: Between 04/2016 and 04/2021, 307 patients (median age, 58 years, range 46 - 66; 58% male) were enrolled. The broad ancestral category was 72% European and 28% non-European. Most patients had newly diagnosed acute leukemia (82%), followed by relapsed acute leukemia (12%) and primary refractory acute leukemia (6%). 73% had acute myeloid leukemia (AML), 22% had acute lymphoblastic leukemia (ALL) and 5% had mixed phenotypic/ambiguous lineage acute leukemia. Of the patients with AML, 21%, 27% and 52% had European LeukemiaNet (ELN) 2017 favorable, intermediate, and adverse risk disease respectively, with significant representation of AML with myelodysplasia-related changes (33%) and therapy-related AML (13%). Of the patients with ALL, 73% had B-ALL and 27% had T-ALL/lymphoblastic lymphoma.
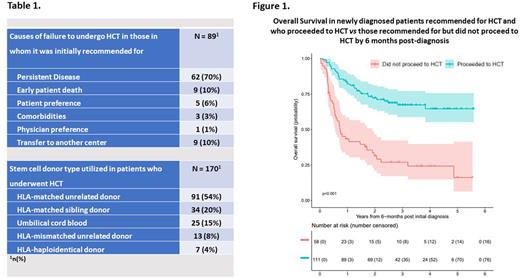
HCT was recommended to 259/307 (85%) of enrolled patients, of whom 170 (66%) underwent transplant. The most common cause for failure to undergo transplant in the 89 (34%) patients in whom it was recommended for was persistent disease (70%), followed by early patient death from regimen toxicity (10%), patient-preference (6%), comorbidities (3%) and physician-preference (1%) (Table 1). Nine patients (10%) were lost to follow up due to transfer to another center. Importantly, no patient failed to proceed to HCT due to the lack of an available donor.
Newly diagnosed patients recommended for HCT but who failed to proceed had significantly inferior OS compared to those recommended for and who proceeded to HCT by 6 months post diagnosis (P < 0.001) (Figure 1). The median time from initiation of therapy to HCT was 112 days (IQR 87 - 145) in newly diagnosed patients. HCT was performed in 10/46 (22%) patients in whom it was not initially recommended for due to subsequently relapsed or persistent disease.
There was high concordance (85%) between the recommended donor and subsequently utilized donor. Utilized donor types comprised 54% HLA-matched unrelated donors, 20% HLA-matched sibling donors and relatively high utilization of alternative graft sources with 15% umbilical cord blood, 8% HLA-mismatched (4-7/8) unrelated donors, and 4% HLA-haploidentical related donors (Table 1).
Conclusion: In this prospective study, the main barrier to HCT was disease control, followed by death due to regimen toxicity. Donor availability was not a barrier to HCT. Thirty-four percent of patients with acute leukemia who were initially recommended for HCT failed to undergo transplant. However, 66% proceeded to transplant and this relatively high HCT rate may relate to early consensus review for transplant eligibility. Based on these data, early transplant referral, development of novel HCT strategies for patients with acute leukemia not achieving remission, and safely improving induction regimens could result in increased utilization of HCT.
Disclosures
Ponce:Seres Therapeutics: Research Funding. Shaffer:Miltenyi Biotec: Research Funding; Gamida Cell: Consultancy; Hansa Biopharma: Consultancy. Politikos:ExcelThera: Membership on an entity's Board of Directors or advisory committees; PrecicionHeor: Honoraria; Merck: Research Funding. Park:Autolus Therapeutics: Consultancy; Artiva Biotherapeutics, Inc.: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Allogene Therapeutics: Membership on an entity's Board of Directors or advisory committees; Affyimmune Therapeutics, Inc.: Consultancy; Juno: Research Funding; Genentech: Research Funding; AstraZeneca: Consultancy; Servier: Consultancy, Other: Provision of Services; Novartis: Consultancy; Kura Oncology: Consultancy; Kite, a Gilead Company: Consultancy; Intellia: Consultancy; Curocell Inc.: Consultancy; Innate Pharma: Consultancy; Bristol-Myers Squibb: Consultancy. Perales:Takeda: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Bellicum: Honoraria; DSMB: Other; MorphoSys: Consultancy, Honoraria; Omeros: Consultancy; Merck: Consultancy; Nektar Therapeutics: Consultancy, Honoraria; Orca Bio: Consultancy; Novartis: Honoraria; Celgene: Honoraria; VectivBio AG: Honoraria; Vor Biopharma: Honoraria; Medigene: Consultancy; Karyopharm: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Cidara Therapeutics: Consultancy; Sellas Life Sciences: Consultancy; Servier: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria. Tallman:UpToDate: Patents & Royalties: Royalties; Ipsen Biopharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Syros Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; Innate Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Biosight: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Membership on an entity's Board of Directors or advisory committees; KAHR-Adv Bd: Membership on an entity's Board of Directors or advisory committees; Orsenix: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Rafael Pharmaceuticals: Research Funding; Glycomimetics: Research Funding; Biosight: Research Funding; Orsenix: Research Funding; Abbvie: Research Funding. Barker:Merck: Research Funding; New York Blood Center: Consultancy; Gamida Cell: Consultancy. Stein:Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Auron Therapeutics: Current equity holder in private company; PinotBio, Bristol Myers Squibb, Jazz Pharmaceuticals, Foghorn Therapeutics, Blueprint Medicines, Gilead Sciences, Janssen Pharmaceuticals: Consultancy; PTC Therapeutics and Syros: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Research Funding; Bayer: Research Funding; Amgen, AbbVie, Seattle Genetics, and Biotheryx: Consultancy. Gyurkocza:Actinium Pharmaceuticals, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal