In this issue of Blood, in a 5-year update of the randomized phase 3 MURANO trial comparing time-limited therapy with venetoclax plus rituximab (VenR) vs bendamustine plus rituximab (BR) for patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL), Seymour et al1 report continued superiority of progression-free survival (PFS) and overall survival (OS) with VenR compared with BR. Most significantly, they provide long-term follow-up for patients who attain peripheral blood undetectable minimal residual disease (uMRD) at end of treatment (EOT). The investigators explored the kinetics of disease relapse for patients who did attain uMRD. They found slower uMRD doubling time in patients treated with VenR vs BR and described 32 patients with uMRD after VenR who remain with uMRD 3 years after EOT. They show that patients with high-risk disease are more likely to relapse to minimal residual disease (MRD) with progression of disease after attaining uMRD. Last, the investigators provided a glimpse into treatment after relapse, with reassuring data showing that re-treatment with a venetoclax-based regimen or a Bruton tyrosine kinase inhibitor (BTKi) led to high-response rates in patients treated with VenR.
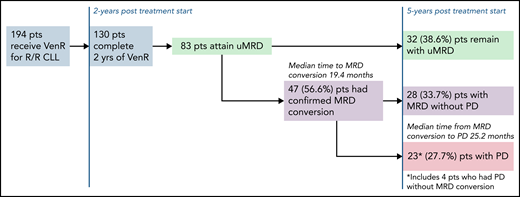
In 2018, the original data from the MURANO trial demonstrating a PFS benefit of VenR compared with BR for R/R CLL were published. No one could deny that the Kaplan-Meier curves for PFS and OS for VenR-treated patients were impressive.2 This breakthrough using time-limited targeted therapy was, and still is, an attractive therapeutic option for patients. Now, we have 5 years of follow-up for this study, and VenR as a treatment regimen for R/R CLL continues to impress with a median PFS of 53.6 months, and a 5-year OS of 82.1%. Of note, only 8 participants were previously treated with a BTKi. Although not surprising given the era in which the trial was initiated, this does limit the ability to generalize to patients treated with prior BTKi. As use of BTKi’s and venetoclax plus obinutuzumab has become more ubiquitous in the frontline setting, further research is needed to determine the efficacy of VenR after these treatments. The MURANO investigators did provide results on the efficacy of the next line of therapy after patients progressed after VenR, with high overall response rates observed for patients treated with either venetoclax-based regimens or a BTKi. In several retrospective analyses, response rates have been reported,3,4 but this is the largest data set reported thus far of a prospective cohort. It will be helpful to see additional follow-up, especially regarding response duration. In the CLL8 trial of frontline fludarabine/cyclophosphamide vs fludarabine/cyclophosphamide/rituximab (FCR), patients with immunoglobulin heavy chain gene (IGHV) mutated disease treated with FCR had a median PFS that was not reached after a median follow-up of 5.9 years.5 In a follow-up study, investigators found that attaining uMRD, defined as ≤1 × 10−4 by flow cytometry in peripheral blood, was prognostic for PFS and OS.6 These findings were among the first to suggest that attaining uMRD may be a valuable surrogate endpoint for survival. However, questions remained as to whether nonchemoimmunotherapy regimens could induce uMRD, and whether the uMRD induced by targeted therapeutics would be equivalent to those induced by chemoimmunotherapy. The MURANO trial and the subsequent frontline CLL14 trial have put these questions to rest for venetoclax/antibody combinations. In both trials, treatment with venetoclax/antibody induced high rates of uMRD, and uMRD predicted long-term PFS. These 5-year data continue to show that attaining uMRD leads to improved PFS, and, similar to CLL14,7 show a slower MRD growth pattern for patients treated with VenR vs chemoimmunotherapy, and critically, improved OS for patients who attain uMRD compared with those that do not. In the long-term follow-up of this group of patients, the investigators report the median time to conversion to MRD from uMRD was 19.4 months, and median time to progression of disease (PD) from MRD conversion was 25.2 months (see figure). Patients at the highest risk of converting to MRD, which are also the same patients who had worse overall PFS and OS, were those patients that had unmutated IGHV, del(17p), or genomic complexity. Although it is reassuring that the time from MRD conversion to relapse is long, based on these data, the question can be raised of whether some patients would benefit from continuous venetoclax, as opposed to time-limited therapy. Although this approach should be studied for select patients, continuous venetoclax may lead to the development of resistance mutations,8 clonal hematopoiesis,9 and cumulative toxicity, all of which would be expected to be less common with time-limited approaches.
Patient disposition after 5-year follow-up of MURANO study for patients who were uMRD at the end of therapy. pts, patients.
Patient disposition after 5-year follow-up of MURANO study for patients who were uMRD at the end of therapy. pts, patients.
These data confirm that uMRD is a viable surrogate endpoint for survival with venetoclax/antibody regimens. Two important questions remain: What is the ideal way to test for MRD, and what is the optimal cut-point for MRD assessment? Both flow cytometry and next-generation sequencing (NGS) have been used in the clinical trial context and are likely equivalent methods when evaluating a depth of ≤1 × 10−4, with MURANO using flow cytometry, and CLL14 NGS. Blood and marrow compartments have also been tested in multiple studies; although there are data to support general concordance between uMRD measured in the peripheral blood and the bone marrow for patients treated with chemoimmunotherapy,10 this concordance has not been solidified with venetoclax-based regimens. Finally, although ≤1 × 10−4 is generally used to define uMRD, NGS sequencing has shown that deeper levels of MRD can be evaluated. However, the clinical consequences of ≤1 × 10−4 vs ≤1 × 10−6 with venetoclax-based regimens have not been identified, as formal comparisons have not been performed. Ultimately, more research is needed to determine which test, where to test, and how deep to test, especially if MRD assessment will be used to alter therapy rather than just for prognosis.
In conclusion, this 5-year follow-up of the MURANO trial continues to show deep and durable responses to VenR in patients with R/R CLL. It further cements the use of MRD testing as a surrogate for survival in patients with R/R disease treated with VenR. Therefore, we would assert that MRD is “the” endpoint for venetoclax/antibody treatment in CLL.
Conflict-of-interest disclosure: A.S.K. has consulted for Abbvie, Beigene, Bristol Myers Squibb, and Janssen. J.A.W. receives research funding from Pharmacyclics, Abbvie, Janssen, Karyopharm, Morphosys, and Schrodinger and has consulted for Abbvie, Pharmacyclics, Janssen, AstraZeneca, Beigene, Genentech, Loxo, and Newave. J.A.W. is a clinical scholar of the Leukemia and Lymphoma Society.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal