TO THE EDITOR:
Measurable residual disease (MRD) determined by multiparameter flow cytometry (MFC), which covers >90% of acute myeloid leukemia (AML) cases,1 has been an important variable for predicting relapse and directing treatment approach selection.1-4 However, relapse is observed in a considerable number (20%-70%) of patients with low/negative MRD levels5 using the cutoff value of 0.1% for MFC-determined MRD.1 Both leukemia-associated immunophenotype (LAIP)1 and “different-from-normal (D-F-N)”6,7 approaches for MRD evaluation (traditional MFC methods) have the limitation of providing false-negative results. The heterogeneity in the immunophenotypes of different AML subtypes,8,9 gains or losses of antigen expression during treatment, and the low detection sensitivity of traditional MFC methods5,6,10 might account for false negatives. Therefore, a novel method for MRD evaluation by MFC is needed to solve these problems.
Several researchers have suggested that assays detecting leukemia stem cells (LSCs) by MFC might increase the sensitivity and predictive value of MRD, which has a false-negative rate of 13% to 30% in patients with AML,11-17 because the chemoresistant reservoir of MRD consists of rare LSCs below the detection threshold of 0.1% to 0.01%. However, few of these studies compared the traditional MFC method with LSC-based MRD assays in terms of, for example, differences in sensitivity16 and the prediction of cumulative incidence of relapse (CIR) and the time from positive MRD to hematological relapse.12 Moreover, these studies are mostly retrospective, with differences in LSC detection panels, and do not include training and validation sets.11-17 Therefore, no definitive conclusion can be drawn regarding the superiority of LSC-based assays to the traditional MFC assay.
Recently, Zeijlemaker et al18 designed a single 8-color LSC detection tube consisting of a cocktail of 6 stable cell surface markers (CD56/CD22/CD11b/CD7/Tim-3/CLL-1), which reportedly facilitates complete and accurate LSC detection in the CD34+CD38− subsets of AML.18 Therefore, we prospectively compared the traditional MFC method and the LSC-based MRD assay by Zeijlemaker et al18 in patients with AML who had received allografts and were randomized into training (n = 180) and validation (n = 180) groups.
A description of the study design can be found in the supplemental methods (supplemental Tables 1-2; supplemental Figures 1-3), available on the Blood Web site. MRD was evaluated by an LSC-based MRD assay18 and the traditional MFC method2 simultaneously. We found that marker expression on CD34+CD38− stem cells remained stable 1 year after transplantation (supplemental Table 3). The median number of events for the LSC assay was 1 000 000 (range, 100 875-2 006 250). In contrast to previous studies,12,13,15 we found that CD34+CD38− LSCs after allograft transplantation could be used for relapse, but not leukemia-free survival (LFS) and overall survival (OS), prediction in patients with AML (supplemental Figure 4; supplemental Tables 4-6). Several factors might account for the differences: (1) differences in the time of MRD detection, (2) differences in therapies between other studies (chemotherapy) and ours (allograft transplantation), and (3) potential differences in regenerated normal autologous CD34+CD38− cells after chemotherapy and allogeneic CD34+CD38− cells following allograft.
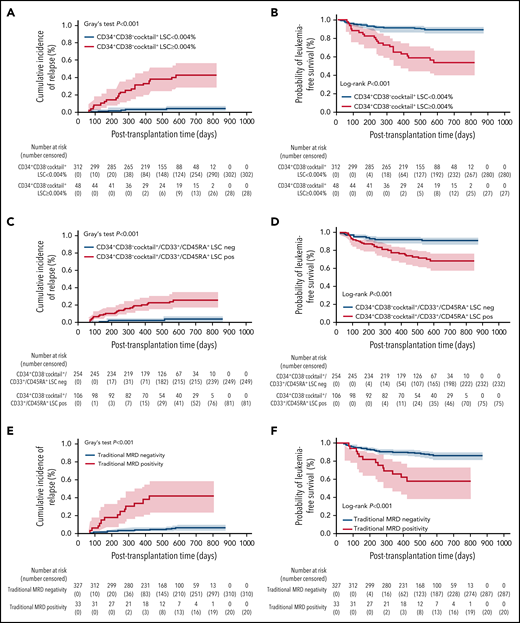
According to a previous study,12 we identified a cutoff value of CD34+CD38−cocktail+ LSCs (0.004%) to divide patients with AML into different relapse risk groups (supplemental Tables 7 and 8; supplemental Figures 5 and 6). Multivariate analysis showed the predictive values of CD34+CD38−cocktail+ LSCs for CIR (P < .001 for all), LFS (P < .001 for all), and OS (P = .004; P < .001) in patients in both the training and the validation sets. In all patients (n = 360, Table 1; supplemental Tables 9-11; Figure 1A-B), compared with the traditional MFC method, the CD34+CD38−cocktail+ LSC-based MRD assay had a high sensitivity (66.7% vs 43.3%), C-index (0.76 vs 0.69), and Youden index (0.58 vs 0.37). The superiority of the CD34+CD38−cocktail+ LSC-based MRD assay to the traditional MFC method (supplemental Figure 7)6,7,10,19 might be related to the self-renewal ability and leukemia initiation capability of CD34+CD38−cocktail+ LSCs (supplemental Table 10; supplemental Figure 8), which might provide enough time for physicians to adopt relapse interventional strategies.20 In addition, the predictive role of the CD34+CD38−cocktail+ LSC-based MRD assay was not affected by other variables, such as pre–hematopoietic stem cell transplantation MRD and mixed chimerism (supplemental Tables 9-11).
Outcomes of patients with AML classified into different subgroups according to posttransplantation MRD using different biomarkers (n = 360)
| . | 2-y CIR . | 2-y NRM . | 2-y LFS . | 2-y OS . |
|---|---|---|---|---|
| Total patients (n = 360) | ||||
| Cases with negative CD34+CD38−cocktail+ LSC | 3.8% | 7.2% | 89.0% | 89.6% |
| (n = 312, group A) | (95% CI, 1.3%-6.3%) | (95% CI, 3.9%-10.5%) | (95% CI, 85.1%-92.9%) | (95% CI, 85.9%-93.9%) |
| Cases with positive CD34+CD38−cocktail+ LSC | 44.3%* | 2.1% | 53.5%* | 62.3%* |
| (n = 48, group B) | (95% CI, 29.2%-59.4%)* | (95% CI, 0%-6.4%) | (95% CI, 38.6%-68.4%)* | (95% CI, 46.8%-77.8%)* |
| Total patients (n = 360) | ||||
| Cases with negative CD34+CD38−cocktail+/CD33+/CD45RA+ LSC† | 2.5% | 6.8% | 90.9% | 92.1% |
| (n = 254, group C) | (95% CI, 0.1%-4.9%) | (95% CI, 3.7%-9.9%) | (95% CI, 87.2%-94.6%) | (95% CI, 88.8%-95.4%) |
| Cases with positive CD34+CD38−cocktail+/CD33+/CD45RA+ LSC | 26.2%‡ | 7.3% | 68.1%‡ | 77.4%‡ |
| (n = 106, group D) | (95% CI, 17.2%-35.2%)‡ | (95% CI, 1.4%-13.2%) | (95% CI, 58.5%-77.7%)‡ | (95% CI, 69.0%-85.8%)‡ |
| Total patients (n = 360) | ||||
| Cases with negative traditional MRD determined by MFC | 7.3% | 7.2% | 85.6% | 87.5% |
| (n = 327, group E) | (95% CI, 3.6%-11.0%) | (95% CI, 4.1%-10.3%) | (95% CI, 80.9%-90.3%) | (95% CI, 82.6%-92.4%) |
| Cases with positive traditional MRD determined by MFC | 44.9%‖ | 0% | 55.1%‖ | 55.6%‖ |
| (n = 33, group F) | (95% CI, 25.1%-64.7%)‖ | (95% CI, 35.9%-74.3%)‖ | (95% CI, 35.2%-76.0%)‖ |
| . | 2-y CIR . | 2-y NRM . | 2-y LFS . | 2-y OS . |
|---|---|---|---|---|
| Total patients (n = 360) | ||||
| Cases with negative CD34+CD38−cocktail+ LSC | 3.8% | 7.2% | 89.0% | 89.6% |
| (n = 312, group A) | (95% CI, 1.3%-6.3%) | (95% CI, 3.9%-10.5%) | (95% CI, 85.1%-92.9%) | (95% CI, 85.9%-93.9%) |
| Cases with positive CD34+CD38−cocktail+ LSC | 44.3%* | 2.1% | 53.5%* | 62.3%* |
| (n = 48, group B) | (95% CI, 29.2%-59.4%)* | (95% CI, 0%-6.4%) | (95% CI, 38.6%-68.4%)* | (95% CI, 46.8%-77.8%)* |
| Total patients (n = 360) | ||||
| Cases with negative CD34+CD38−cocktail+/CD33+/CD45RA+ LSC† | 2.5% | 6.8% | 90.9% | 92.1% |
| (n = 254, group C) | (95% CI, 0.1%-4.9%) | (95% CI, 3.7%-9.9%) | (95% CI, 87.2%-94.6%) | (95% CI, 88.8%-95.4%) |
| Cases with positive CD34+CD38−cocktail+/CD33+/CD45RA+ LSC | 26.2%‡ | 7.3% | 68.1%‡ | 77.4%‡ |
| (n = 106, group D) | (95% CI, 17.2%-35.2%)‡ | (95% CI, 1.4%-13.2%) | (95% CI, 58.5%-77.7%)‡ | (95% CI, 69.0%-85.8%)‡ |
| Total patients (n = 360) | ||||
| Cases with negative traditional MRD determined by MFC | 7.3% | 7.2% | 85.6% | 87.5% |
| (n = 327, group E) | (95% CI, 3.6%-11.0%) | (95% CI, 4.1%-10.3%) | (95% CI, 80.9%-90.3%) | (95% CI, 82.6%-92.4%) |
| Cases with positive traditional MRD determined by MFC | 44.9%‖ | 0% | 55.1%‖ | 55.6%‖ |
| (n = 33, group F) | (95% CI, 25.1%-64.7%)‖ | (95% CI, 35.9%-74.3%)‖ | (95% CI, 35.2%-76.0%)‖ |
CI, confidence interval; NRM, nonrelapse mortality.
P < .001 compared with group A.
The LSC-based MRD positivity was defined as patients with either CD34+CD38−cocktail+ LSC ≥ 0.004%, or CD34+CD38−CD33+ LSC ≥ 0.025%, or CD34+CD38−CD45RA+ LSC ≥ 0.007%.
P < .01 compared with group C.
P < .001 compared with group E.
Relationship between posttransplantation LSC-based MRD assay or traditional assay detected by MFC and transplant outcomes for patients with AML who underwent allo-SCT (n = 360). Competing risk model and Kaplan-Meier estimates of relapse and LFS, respectively, according to posttransplantation CD34+CD38−cocktail+ LSCs (A-B), posttransplant CD34+CD38−cocktail+/CD33+/CD45RA+ LSCs (C-D), and posttransplantation traditional MRD (E-F). allo-SCT, allogeneic stem cell transplantation; neg, negative; pos, positive.
Relationship between posttransplantation LSC-based MRD assay or traditional assay detected by MFC and transplant outcomes for patients with AML who underwent allo-SCT (n = 360). Competing risk model and Kaplan-Meier estimates of relapse and LFS, respectively, according to posttransplantation CD34+CD38−cocktail+ LSCs (A-B), posttransplant CD34+CD38−cocktail+/CD33+/CD45RA+ LSCs (C-D), and posttransplantation traditional MRD (E-F). allo-SCT, allogeneic stem cell transplantation; neg, negative; pos, positive.
In the total patient group, we found that the combined traditional MFC method/CD34+CD38−cocktail+ LSC-based MRD assay had profound predictive significance for CIR and survival (supplemental Tables 10 and 13). The superiority of the CD34+CD38−cocktail+ LSC-based MRD assay to the traditional MFC method in predicting relapse was observed in adult patients, but not in pediatric patients (supplemental Tables 14-18). For patients with leukemia-specific genes or gene mutations (n = 184), combinatory analyses of LSC and molecular MRD showed the superiority of the CD34+CD38−cocktail+ LSC/molecular MRD to each of these 2 methods in predicting relapse (supplemental Tables 19-22). In addition, 180 days posttransplant seems to be crucial for clinical prediction (supplemental Tables 23-24).
According to other studies,18,21,22 we further identified cutoff values of 0.025% and 0.007% for CD34+CD38−CD33+ LSCs and CD34+CD38−CD45RA+ LSCs, respectively, in predicting the CIR of patients with AML (n = 360, Table 1; supplemental Tables 25-27; Figure 1C-D). The patients with positive LSCs determined using the CD34+CD38−cocktail+, CD34+CD38−CD33+, or CD34+CD38−CD45RA+ immunophenotypes experienced higher CIR (P < .001) and lower LFS (P < .001) and OS (P = .001) than patients with negative LSCs (Table 1). Despite the high sensitivity (83.3% vs 43.3%) of the abovementioned LSC-based MRD method compared with the traditional MFC method, the specificity was low (75.5% vs 93.9%) (supplemental Table 28). Overall, we found that the CD34+CD38−cocktail+ LSC-based MRD method was better than other LSC marker-based MRD assays for relapse prediction (supplemental Tables 10 and 28).
Subgroup analysis (n = 325) of patients with a CD34+ leukemia cell phenotype11,23 showed that, compared with the traditional MFC method, the CD34+CD38−cocktail+ LSC-based MRD method had a high sensitivity (76.0% vs 48.0%), C-index (0.81 vs 0.71), and Youden index (0.67 vs 0.41) (supplemental Tables 29 and 30; supplemental Figures 9 and 10). Contrary to a previous study,12 we further confirmed the superiority of the CD34+CD38−cocktail+ LSC-based MRD assay, including its sensitivity and median time from positive MRD to hematological relapse (supplemental Figure 7), to the traditional assay.6,7,10
In contrast to the traditional MFC LAIP MRD assay,6,7,10 we previously showed that the false-negative rate was 8.3% for CIR prediction based on the traditional MFC method after allografting in patients with AML.24 In the present study, the false-negative rate ranged from 2.5% to 3.8% for the CD34+CD38−cocktail+ LSC-based MRD method. Although there is a lack of comparability between different studies,5-7,24 data reported by others25 and us suggest a lower false-negative rate in patients with AML when the LSC-based MRD method is used for relapse prediction than when the traditional MFC method is used.
The limitations of our study include the following. First, the cocktail panel for LSCs applied only to patients with AML with a CD34+ leukemia cell phenotype.18 Therefore, a novel single-tube panel including CD117 designed for some patients with MRD characterized by CD117+CD34− phenotype relapse is needed. Second, multicenter prospective studies are warranted to confirm the superiority of the CD34+CD38−cocktail+ LSC-based MRD method to the traditional MFC method for AML relapse prediction. Moreover, the potential impact of clonal hematopoiesis and clonal emergence in AML kinetics as well as LAIP at onset should be considered.
Overall, we first confirmed the superiority of the CD34+CD38−cocktail+ LSC-based MRD assay, such as its higher sensitivity and longer time from MRD positivity to relapse, to the traditional MFC method for outcome prediction in patients with AML with randomized training and validation sets (supplemental Table 10; Figure 1E-F). Our findings suggest that the CD34+CD38−cocktail+ LSC-based MRD method should be used routinely for AML relapse prediction and prognosis stratification.
Acknowledgments
The authors thank all of the faculty members who participated in these studies. They also thank American Journal Experts (www.aje.com) for assistance in editing the manuscript.
This work was partly supported by the National Key Research and Development Program of China grant no. 2017YFA0104500, Beijing Municipal Science and Technology Commission grant Z181100009618032, National Natural Science Foundation of China grant no. 82070185, Peking University Clinical Scientist Program grant no. BMU2019LCKXJ003, and the Sino-Russian Mathematics Foundation SRMCF 2021.
Authorship
Contribution: Y.-J.C. and X.-J.H. designed the study; Y.-J.C. and S.-Q.L. collected the data; H.C., Y.-H.C., Y.-J.C., W.H., X.-J.H., K.-Y.L., M.L., S.-Q.L., Y.-R.L., X.-D.M., Y.-Q.S., F.-F.T., F.-R.W., Y.W., L.-P.X., C.-H.Y., and X.-H.Z. analyzed the data and drafted the manuscript; and all authors contributed to data interpretation, manuscript preparation, and the approval of the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ying-Jun Chang and Xiao-Jun Huang, Peking University Institute of Hematology, Peking University People’s Hospital, No 11 Xizhimen South St, Beijing 100044, China; e-mail: rmcyj@bjmu.edu.cn and xjhrm@medmail.com.cn.
Presented in part as an oral presentation at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
Data from this paper may be acquired by contacting the corresponding authors, Ying-Jun Chang and Xiao-Jun Huang through e-mail.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
This study was registered as #ChiCTR1800016458 at http://www.chictr.org.cn/showproj.aspx?proj=27944.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal