Key Points
Microlyse is noninferior to rh-tPA when AIS is driven by fibrin-rich thrombi, but superior in AIS driven by platelet-rich thrombi.
VWF-targeted plasminogen activation overcomes the intrinsic resistance of platelet-rich thrombi against rh-tPA–mediated thrombolysis.
Abstract
Recombinant human tissue plasminogen activator (rh-tPA) is an important thrombolytic agent for treatment of acute ischemic stroke. It requires fibrin binding for plasminogen activation. In contrast, Microlyse, a novel thrombolytic agent, requires von Willebrand factor (VWF) binding for plasminogen activation. We compared rh-tPA with Microlyse, administered 20 minutes after inducing thrombosis, in 2 randomized blinded acute ischemic stroke mouse models. Thrombosis was induced in the middle cerebral artery with different experimental triggers. Where thrombin infusion generates fibrin-rich thrombi, topical FeCl3 application generates platelet-rich thrombi. In the fibrin-rich model, both rh-tPA and Microlyse increased cortical reperfusion (determined by laser speckle imaging) 10 minutes after therapy administration (35.8 ± 17.1%; P = .001 39.3 ± 13.1%; P < .0001; 15.6 ± 7.5%, respectively, vs vehicle). In addition, both thrombolytic agents reduced cerebral lesion volume (determined by magnetic resonance imaging) after 24 hours (18.9 ± 11.2 mm3; P = .033; 16.1 ± 13.9 mm3; P = .018; 26.6 ± 5.6 mm3, respectively, vs vehicle). In the platelet-rich model, neither rh-tPA nor Microlyse increased cortical reperfusion 10 minutes after therapy (7.6 ± 8.8%; P = .216; 16.3 ± 13.9%; P = .151; 10.1 ± 7.9%, respectively, vs vehicle). However, Microlyse, but not rh-tPA, decreased cerebral lesion volumes (13.9 ± 11.4 mm3; P < .001; 23.6 ± 11.1 mm3; P = .188; 30.3 ± 10.9 mm3, respectively, vs vehicle). These findings support broad applicability of Microlyse in ischemic stroke, irrespective of the thrombus composition.
Introduction
IV administration of recombinant human tissue plasminogen activator (rh-tPA [alteplase]) is the only approved thrombolytic treatment for acute ischemic stroke (AIS). Nonetheless, its efficacy, based on survival in the absence of disability, is estimated at less than 35%, with a persistent risk for intracranial bleeding of 7%.1-6 tPA is an endogenous protease that requires binding to fibrin to activate plasminogen into plasmin, which then actively degrades fibrin. Plasmin can also cleave von Willebrand factor (VWF),7 but none of the known plasminogen activators can activate plasminogen in a VWF-dependent manner.
The limited efficacy of rh-tPA to prevent ischemic injury is also known as “rh-tPA resistance.”8-10 This phenomenon could be explained by new insights in clot architecture that challenge the assumption that fibrin is always available for rh-tPA binding in thrombi that cause AIS. First, experimental models for thrombosis after vascular injury in vivo have shown that fibrin forms in the thrombus core, near the site of injury, whereas the shell, reaching into the vessel lumen, is fibrin poor.11,12 In this context, fibrin is not accessible to intravascular thrombolytic agents. Second, histopathological examination of thrombectomized human thrombi (by definition excluding microvascular thrombi) showed large variability in the fibrin content.8,13,14 Combined, these findings suggest that targeting nonfibrin components may hold value in the treatment of AIS.8 VWF is a critical component in thrombus formation.6 Indeed, degrading VWF by administration of ADAMTS13, at either supraphysiological levels15 or via a gain-of-function mutant,16 decreased cerebral lesion volumes in preclinical AIS models. However, ADAMTS13 cannot degrade fibrin,17 limiting its applicability to VWF-dependent thrombi.15
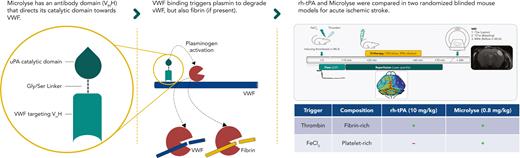
In current clinical practice, the clot composition is unknown before treatment. Consequently, the availability of fibrin that determines rh-tPA efficacy is not guaranteed. In an attempt to improve the efficacy of thrombolytic therapy, we recently developed Microlyse. Microlyse is a single polypeptide consisting of a VWF-targeting nanobody and the catalytic domain of urokinase plasminogen activator. Upon binding VWF, Microlyse initiates localized plasminogen activation.18 We demonstrated its efficacy in a preclinical model for thrombotic thrombocytopenic purpura without increasing bleeding risk. Thrombotic thrombocytopenic purpura is a thrombotic microangiopathy with a dominant role for VWF and a minor, if any, role for fibrin. Because plasmin can degrade both fibrin as well as VWF, we here set out to compare the efficacy of Microlyse and rh-tPA in both a fibrin- and a platelet-rich mouse model of AIS. Based on the ability to decreased ischemic lesion volumes, it was previously shown that the fibrin-rich model is rh-tPA sensitive, whereas the platelet-rich model is rh-tPA resistant but ADAMTS13 sensitive (ie, VWF dependent).15,19
Study design
The experimental design is shown in Figures 1A and 2A, and an expanded description of the Methods is supplied in the supplemental Data (available on the Blood website).
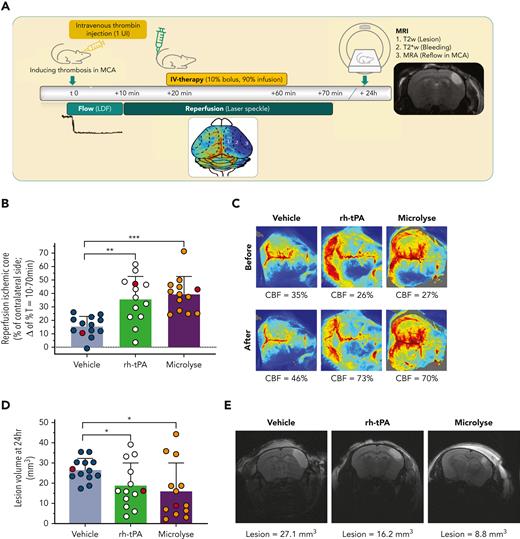
Fibrin-rich thrombosis model (thrombin induced; rh-tPA sensitive). (A) AIS was induced by intravascular injection of thrombin into the MCA. Clot formation was considered to be stable when blood flow in the MCA was zero for 10 minutes. Cortical perfusion over the entire dorsal brain area was measured via speckle imaging between T = 10 minutes and T = 70 minutes. Treatment was given at T = 20 minutes as a 10% bolus and 90% infusion over 40 minutes. MRI was performed after 24 hours to assess lesion volumes (T2 weighted), possible hemorrhage (T2∗-weighted), and MCA recanalization (MR angiography). (B) Bar graph (mean ± standard deviation) displaying the percentage of reperfusion of the ischemic core, calculated as difference between levels of perfusion 10 minutes after treatment (T = 70 minutes) and 10 minutes before treatment (T = 10 minutes). From individual mice indicated as red circles (reflecting median values), images are presented hereafter. (C) Representative laser speckle flow images (from subjects indicated at red circles in panel B) before and after treatment from a single mouse per group. Reperfusion is visible when the blue hemisphere (bottom half of each image) before treatment is turning green, yellow, or red after treatment. The mean CBF levels in the ischemic core (expressed as percent of the unaffected hemisphere) are shown below the images. (D) Bar graph (mean ± standard deviation) displaying lesion volumes, calculated from a T2-weighted MRI. From individual mice indicated as red circles (reflecting median values), images are presented hereafter. (E) Representative T2-weighted images of coronal mouse brain slices displaying the hyperintense lesion area in a single mouse per group (indicated as red circles in D). Lesion volume values are shown below the images. Asterisks indicate significance levels as measured by Mann-Whitney U tests: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBF, cerebral blood flow.
Fibrin-rich thrombosis model (thrombin induced; rh-tPA sensitive). (A) AIS was induced by intravascular injection of thrombin into the MCA. Clot formation was considered to be stable when blood flow in the MCA was zero for 10 minutes. Cortical perfusion over the entire dorsal brain area was measured via speckle imaging between T = 10 minutes and T = 70 minutes. Treatment was given at T = 20 minutes as a 10% bolus and 90% infusion over 40 minutes. MRI was performed after 24 hours to assess lesion volumes (T2 weighted), possible hemorrhage (T2∗-weighted), and MCA recanalization (MR angiography). (B) Bar graph (mean ± standard deviation) displaying the percentage of reperfusion of the ischemic core, calculated as difference between levels of perfusion 10 minutes after treatment (T = 70 minutes) and 10 minutes before treatment (T = 10 minutes). From individual mice indicated as red circles (reflecting median values), images are presented hereafter. (C) Representative laser speckle flow images (from subjects indicated at red circles in panel B) before and after treatment from a single mouse per group. Reperfusion is visible when the blue hemisphere (bottom half of each image) before treatment is turning green, yellow, or red after treatment. The mean CBF levels in the ischemic core (expressed as percent of the unaffected hemisphere) are shown below the images. (D) Bar graph (mean ± standard deviation) displaying lesion volumes, calculated from a T2-weighted MRI. From individual mice indicated as red circles (reflecting median values), images are presented hereafter. (E) Representative T2-weighted images of coronal mouse brain slices displaying the hyperintense lesion area in a single mouse per group (indicated as red circles in D). Lesion volume values are shown below the images. Asterisks indicate significance levels as measured by Mann-Whitney U tests: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBF, cerebral blood flow.
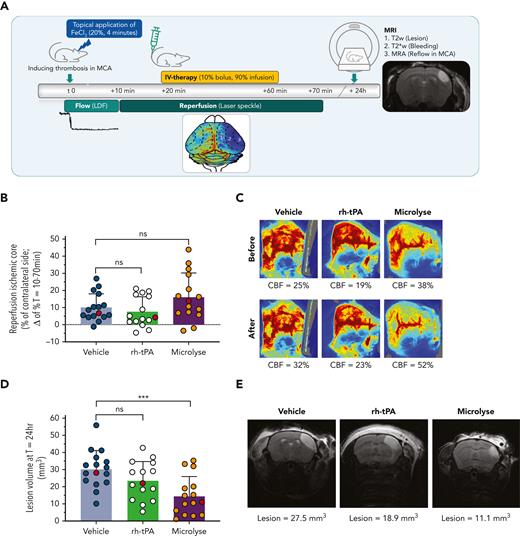
Platelet-rich thrombosis model (FeCl3 induced; rh-tPA resistant). (A) AIS was induced by topical application of FeCl3 on the MCA. (B) Bar graph (mean ± standard deviation) displaying the percentage of reperfusion of the ischemic core, calculated as difference between levels of perfusion 10 minutes after treatment (T = 70 minutes) and 10 minutes before treatment (T = 10 minutes). From individual mice indicated as red circles, images are presented hereafter. (C) Representative laser speckle flow images (from subjects indicated at red circles in panel B) before and after treatment from a single mouse per group. Reperfusion seems to be absent as the blue and green hemisphere (bottom half of each image) before treatment remains blue and green after treatment. The mean CBF levels in the ischemic core (expressed as percent of the unaffected hemisphere) are shown below the images. (D) Bar graph (mean ± standard deviation) displaying lesion volumes, calculated from a T2-weighted MRI. From individual mice indicated as red circles (reflecting median values), images are presented hereafter. (E) Representative T2-weighted images of coronal mouse brain slices displaying the hyperintense lesion area in a single mouse with median lesion volume per group (indicated as red circles in panel D). Lesion volume values are shown below the images. Asterisks indicate significance levels as measured by Mann-Whitney U tests: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBF, cerebral blood flow.
Platelet-rich thrombosis model (FeCl3 induced; rh-tPA resistant). (A) AIS was induced by topical application of FeCl3 on the MCA. (B) Bar graph (mean ± standard deviation) displaying the percentage of reperfusion of the ischemic core, calculated as difference between levels of perfusion 10 minutes after treatment (T = 70 minutes) and 10 minutes before treatment (T = 10 minutes). From individual mice indicated as red circles, images are presented hereafter. (C) Representative laser speckle flow images (from subjects indicated at red circles in panel B) before and after treatment from a single mouse per group. Reperfusion seems to be absent as the blue and green hemisphere (bottom half of each image) before treatment remains blue and green after treatment. The mean CBF levels in the ischemic core (expressed as percent of the unaffected hemisphere) are shown below the images. (D) Bar graph (mean ± standard deviation) displaying lesion volumes, calculated from a T2-weighted MRI. From individual mice indicated as red circles (reflecting median values), images are presented hereafter. (E) Representative T2-weighted images of coronal mouse brain slices displaying the hyperintense lesion area in a single mouse with median lesion volume per group (indicated as red circles in panel D). Lesion volume values are shown below the images. Asterisks indicate significance levels as measured by Mann-Whitney U tests: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CBF, cerebral blood flow.
Thrombosis was induced at the M2 segment of the right middle cerebral artery (MCA) of OF1 mice under anesthesia. Experiments were performed in a randomized and blinded manner. Fibrin-rich thrombosis19 (n = 12-13 mice per group) was induced by the luminal injection of 1 UI murine thrombin (Diagnostica Stago, Asnières sur Seine, France).20 Platelet-rich thrombosis (n = 15-16 mice per group) was induced by the topical application of FeCl3 (20%, 4 minutes).10,21 Laser Doppler flowmetry was started to confirm full MCA segment occlusion. At T = 10 minutes, baseline cortical perfusion was determined via laser speckle imaging (FLPI-2, Moor Instruments, Axminster, United Kingdom). Between T = 20 minutes and T = 60 minutes, vehicle, 0.8 mg/kg Microlyse, or 10 mg/kg rh-tPA was administered by bolus injection (10% of total dose) and infusion via the tail vein (90% of total dose). Doses were selected based on dose range efficacy studies in a VWF-driven model for thrombotic microangiopathy.18 For rh-tPA, the highest effective dose without hemorrhagic transformation in mice was selected.22 At T = 70 minutes, cortical perfusion was remeasured. Reperfusion data indicate the difference in perfusion before and after therapy. At T = 24 hours, mice were anesthetized and subjected to magnetic resonance imaging examination as described23 to measure cerebral lesion volume (T2-weighted imaging), to detect cerebral hemorrhage (T2∗-weighted imaging), and to assess MCA recanalization (time-of-flight angiography). Lesion volume, expressed in cubic millimeters, was measured from the cortical areas displaying hyperintensity on 20 two-dimensional images. Cerebral hemorrhage per animal, identified as hypointensity, was scored as present versus absent. MCA recanalization was scored in a blinded manner as full recanalization (full MCA was visible), partial recanalization (MCA was poorly visible, or sections were missing), or no recanalization (distal part invisible). Data are presented as mean ± standard deviation and treatment groups were compared with vehicle using the Wilcoxon Mann-Whitney U test. Differences were considered significant when P was <.05.
Results and discussion
In the fibrin-rich AIS model, spontaneous reperfusion (T = 70 minutes) in vehicle-treated mice was 15.6 ± 7.5% (Figure 1B). Reperfusion levels were higher after rh-tPA or Microlyse administration (35.8 ± 17.1%; P = .001; 39.3 ± 13.1%; P < .0001, respectively) (Figure 1B-C). After 24 hours, all mice showed full MCA recanalization. In addition, lesion volumes were smaller after administration of rh-tPA or Microlyse (18.9 ± 11.2 mm3; P = .033; 16.1 ± 13.9 mm3; P = .018; 26.6 ± 5.6 mm3, respectively, vs vehicle) (Figure 1D-E). Intracerebral hemorrhage was detected in 1 mouse (rh-tPA group).
In the platelet-rich AIS model, spontaneous reperfusion (T = 70 minutes) in vehicle-treated mice was 10.1 ± 7.9% (Figure 2B). Comparable reperfusion was measured after rh-tPA or Microlyse administration (7.6 ± 8.8%; P = .216; 16.3 ± 13.9%; P = .151, respectively) (Figure 2B-C). After 24 hours, 25% of the vehicle-treated mice showed MCA recanalization, suggesting that this model results in more severe thrombotic occlusion than is seen in the fibrin-rich model. By comparison, MCA recanalization was observed in 62% of Microlyse-treated mice and 92% of rh-tPA-treated mice (P < .001; P = .073, respectively, vs vehicle). However, lesion volumes were only smaller after administration of Microlyse, but not after rh-tPA administration (13.9 ± 11.4 mm3; P < .001; 23.6 ± 11.1 mm3; P = .188; 30.3 ± 10.9 mm3, respectively, vs vehicle) (Figure 2D-E). No intracerebral hemorrhage was detected with either treatment in this model. The observed variation in reperfusion and lesion volumes, as well as the observation that individual mice receiving vehicle have better outcomes than mice receiving thrombolytic therapy, is in line with previous studies in the field.10,15,16,20
The presence and accessibility of fibrin are critical determinants in the efficacy of rh-tPA in mouse models for stroke.13,19 In contrast, the efficacy of Microlyse is critically dependent on VWF binding.18 Indeed, rh-tPA increased reperfusion (T = 70 minutes) and decreased cerebral lesion volumes (T = 24 hours) in a fibrin-rich AIS model. The same holds true for Microlyse, supporting histopathological findings that VWF is present in all thrombi (average 30%; minimally 5%),8 even when these are fibrin rich. In sharp contrast, in a platelet-rich AIS model, neither agent increased early reperfusion (T = 70 minutes). This finding suggests either that thrombus formation is still ongoing between the moment of therapy administration and the time of perfusion measurements (T = 70 minutes) or, alternatively, that these thrombi are more robust than those induced via thrombin. At T = 24 hours after AIS induction, we observed 2 seemingly contradictive results. First, although Microlyse did not improve reperfusion at T = 70 minutes, it decreased lesion volumes (T = 24 hours) nonetheless. This finding suggests that Microlyse acts in the time frame in between both measurements.24 Second, rh-tPA showed better MCA recanalization (T = 24 hours) than Microlyse (92% vs 62%, respectively), but was not associated with a decreased lesion volume. This either suggest that Microlyse triggers (partial) MCA recanalization earlier than rh-tPA, or that Microlyse, but not rh-tPA, removes secondary distal microthrombi. Future studies will have to examine the performance of Microlyse in dose-escalation effects and thrombolysis-related bleeding in models of hemorrhagic transformation.25-27 Altogether, Microlyse’s ability to degrade both tPA-sensitive and tPA-resistant thrombi supports its further development as promising thrombolytic treatment for AIS.
Acknowledgments
The authors thank Strok@lliance (Caen, France), and specifically Annelise Letourneur and Nicolas Violle for performing the in vivo experiments and analyses.
This study was supported by Stichting Life Sciences Health–TKI (Health∼Holland) LSH PPP Allowance grant 2020 (#TKI2023) (C.M.) and the TTW section of the Netherlands Organization for Scientific Research (NWO, 2019/TTW/00704802) (S.d.M.).
Authorship
Contribution: M.V.A.v.M., S.d.M., K.V., D.V., R.M.D., and C.M. conceived and designed the study; E.M.v.L., C.J., and T.B. performed (pilot) experiments; and M.V.A.v.M., S.d.M., H.B.v.d.W., R.M.D., and C.M. wrote the manuscript.
Conflict-of-interest disclosure: C.M. has been a speaker for Shire/Takeda on topics unrelated to this work. C.M., M.V.A.v.M., K.V., and S.d.M. are founders of TargED Biopharmaceuticals BV, a biotech spinout company of University Medical Center Utrecht (to develop Microlyse based upon the WO2019185723 A1 patent). C.M. and S.d.M. participate in revenue sharing as inventors through the commercialization arm of the University Medical Center Utrecht. D.V. is scientific advisor to Strok@lliance (Caen, France). H.B.v.d.W. serves as a consultant to Bayer and TargED Biopharmaceuticals BV. The remaining authors declare no competing financial interests.
Correspondence: Coen Maas, Central Diagnostic Laboratory Research, University Medical Center Utrecht and Utrecht University, Heidelberglaan 100, Room G.03.646, 3584 CX Utrecht, The Netherlands; e-mail: cmaas4@umcutrecht.nl.
References
Author notes
Data are available on request from the corresponding author, Coen Maas (cmaas4@umcutrecht.nl).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
M.V.A.v.M. and S.d.M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal