TO THE EDITOR:
Persistent isolated mild thrombocytopenia (PIMT, defined here as platelet count 100-149 × 109/L) is a common reason for hematology referral. No guidelines exist directing management of PIMT, and scant data exist to guide prognostic discussion of the risks of developing more defined hematologic pathology, such as immune thrombocytopenia (ITP) or hematologic neoplasia.1,2 Without a comprehensive evaluation including expensive molecular testing and bone marrow biopsy, diagnoses of acquired clonal disorders, hereditary thrombocytopenia syndromes, and idiopathic cytopenia of undetermined significance cannot be made or ruled out, and no reliable testing exists to confirm autoimmune platelet destruction. Therefore, management may vary from reassurance only to interval platelet count monitoring to comprehensive evaluation at the time of referral.
Current ITP practice guidelines from the American Society of Hematology (ASH)1 and International Consensus Report,3 along with International Working Group definitions,4 maintain a relatively arbitrary platelet count threshold for ITP diagnosis of <100 × 109/L. The justification for this includes previously reported “normal” counts between 100 and 149 × 109/L in certain populations,5-7 physiologic reductions in pregnancy,6,8 and, most importantly, the findings of a 2006 observational cohort study of adults with platelet counts between 100 and 150 × 109/L of unknown etiology.9 This study found a 6.9% 10-year probability of ITP development in these patients, although the median follow-up was just 5.3 years.9 Given that many patients with PIMT are relatively young, with several decades of being at risk for developing more defined hematologic disease, the goal of the present study was to evaluate the risk of developing hematologic disease—specifically ITP or hematologic neoplasia—in adults with PIMT of unknown etiology over a much longer follow-up.
Patients were identified for screening via queries of the Mass General Brigham Research Patient Data Registry and the study was approved by the Institutional Review Board of Mass General Brigham Healthcare (approval 2018000964/PHS). Database queries were submitted to identify patients aged 18-65 years with platelet counts measured between 100 and 149 × 109/L ≥3 times from 1995 to 2004 without diagnoses or use of medications known to cause thrombocytopenia (supplemental Figure 1, available on the Blood website), who were referred to a hematologist for evaluation at Massachusetts General Hospital. Hematologist referral was required to ensure clinical evaluation by a qualified specialist concluding no diagnosed etiology for thrombocytopenia (thereby allowing unknown etiology designation; given the era, molecular testing was not performed). All patients identified then underwent manual chart review; only patients with persistent isolated platelets 100-149 × 109/L, no prior count <100 × 109/ L, and no identified thrombocytopenia etiology on hematologist evaluation who additionally had consistent, continuous follow-up at our institution for ≥8 years were included. All included patients (PIMT group) were matched at a 1:4 ratio with healthy control subjects with a normal platelet count and no thrombocytopenia history (healthy subject group) according to age, sex, and ethnicity. To avoid immortal time bias, time zero, the specification of the eligibility criteria, and the start of follow up were synchronized for PIMT and healthy subject groups (described in supplemental Methods). Manual chart review was then undertaken to collect detailed patient information and hematologic outcomes for all patients in both groups.
The primary outcome was progression to defined hematologic disease known to cause thrombocytopenia (heretofore referred to simply as “hematologic disease”). Secondary outcomes included ITP diagnosis (per ASH criteria1) and hematologic neoplasia diagnosis (per World Health Organization [WHO] classification) or other defined bone marrow pathology. Time-to-event analyses modeling risk of developing these outcomes in the PIMT patients and matched healthy subjects controlling for patient age were performed using competing-risks regression (method of Fine and Gray, with death as the competing risk).
Database queries returned 61 979 patients meeting platelet count criteria; after applying ICD code filters, machine-learning phenotypes using natural language-processing algorithms, and filtering for hematologist evaluation, 367 patients remained (supplemental Figure 1). Manual chart review confirmed 91 patients with PIMT of unknown etiology who were included in the cohort (median [range] platelet count 134 [100-149]); 364 healthy subjects with normal platelet counts were then matched and included. Baseline characteristics, including sex, age, race, and body mass index were balanced between the 2 groups (Table 1).
Baseline characteristics of each cohort
| Characteristic . | Healthy subject group (n = 364) . | Persistent isolated mild thrombocytopenia group (n = 91) . |
|---|---|---|
| Age, mean (range), y | 55.2 (19-69) | 55.0 (33-74) |
| Sex, n (%) | ||
| Male | 99 (27) | 29 (32) |
| Female | 265 (73) | 62 (68) |
| Race/ethnicity, n (%) | ||
| Asian | 10 (3) | 0 (0) |
| Black/African American | 58 (16) | 12 (14) |
| Hispanic | 10 (3) | 3 (3) |
| White | 274 (75) | 66 (75) |
| Other | 9 (3) | 7 (8) |
| Alcohol use∗, n (%) | ||
| Yes | 178 (49) | 34 (37) |
| No | 138 (38) | 48 (53) |
| Missing | 48 (13) | 9 (9) |
| Body mass index, mean (IQR) | 27.9 (23.9-32.8) | 28.1 (23.1-31.2) |
| Hemoglobin, median (IQR) | 13.5 (12.5-14.6) | 12.6 (11.3-13.9) |
| White blood cell count, median (IQR) | 7.4 (6.2-9.35) | 7.3 (5.4-9.9) |
| Platelet, median (IQR) | 262 (222-315) | 134 (118-146) |
| Characteristic . | Healthy subject group (n = 364) . | Persistent isolated mild thrombocytopenia group (n = 91) . |
|---|---|---|
| Age, mean (range), y | 55.2 (19-69) | 55.0 (33-74) |
| Sex, n (%) | ||
| Male | 99 (27) | 29 (32) |
| Female | 265 (73) | 62 (68) |
| Race/ethnicity, n (%) | ||
| Asian | 10 (3) | 0 (0) |
| Black/African American | 58 (16) | 12 (14) |
| Hispanic | 10 (3) | 3 (3) |
| White | 274 (75) | 66 (75) |
| Other | 9 (3) | 7 (8) |
| Alcohol use∗, n (%) | ||
| Yes | 178 (49) | 34 (37) |
| No | 138 (38) | 48 (53) |
| Missing | 48 (13) | 9 (9) |
| Body mass index, mean (IQR) | 27.9 (23.9-32.8) | 28.1 (23.1-31.2) |
| Hemoglobin, median (IQR) | 13.5 (12.5-14.6) | 12.6 (11.3-13.9) |
| White blood cell count, median (IQR) | 7.4 (6.2-9.35) | 7.3 (5.4-9.9) |
| Platelet, median (IQR) | 262 (222-315) | 134 (118-146) |
IQR, Interquartile range.
As assessed at initial evaluation (time zero).
The median (range) follow-up duration was 20.5 (8.2-32.7) years and 20.1 (9.0-33.3) years in the PIMT and healthy subject groups, respectively. In the PIMT group, 28 patients (30.8%) were diagnosed with hematologic disease, compared with 7 (1.9%) in the healthy subject group. This included 17 patients (18.7%) vs 1 patient (0.3%) diagnosed with ITP, and 13 patients (14.3%) vs 6 patients (1.7%) diagnosed with hematologic neoplasia (2 patients were diagnosed with both ITP and hematologic neoplasia in the PIMT group). Of the patients with hematologic neoplasia in the PIMT group, diagnoses included myelodysplastic syndrome (8 patients), myeloproliferative neoplasm (2 patients), and non-Hodgkin lymphoma (3 patients); in healthy subjects, diagnoses included non-Hodgkin lymphoma (3 patients), multiple myeloma (1 patient), acute myeloid leukemia (1 patient), and clonal cytopenia of undetermined significance (1 patient).
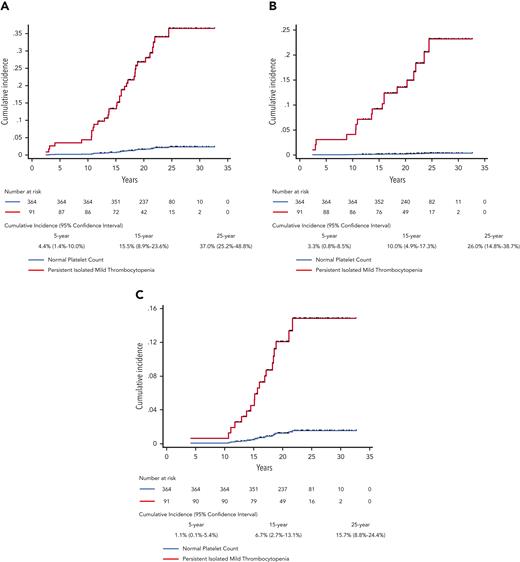
In the PIMT group, the 15-year cumulative incidence of developing hematologic disease was 15.5% (95% CI, 8.9%-23.6%), and the 25-year cumulative incidence of developing hematologic disease was 37.0% (95% CI, 25.2%-48.8%), Figure 1A. The 15-year and 25-year cumulative incidences of development of ITP were 10.0% and 26.0% (Figure 1B), and these incidences for development of hematologic neoplasia were 6.7% and 15.7% (Figure 1C). In competing-risks regression controlling for age, PIMT patients had a significantly higher risk of developing hematologic disease than did healthy subjects (subhazard ratio [SHR], 18.99; 95% confidence interval [CI], 8.39-42.96; P < .001). This included a higher risk of developing both hematologic neoplasia (SHR, 10.33; 95% CI, 3.81-27.99; P < .001) and ITP, although the risk of ITP was difficult to accurately estimate because of the small number of healthy subjects developing ITP (SHR, 71.09; 95% CI, 39.38-538.70; P < .001). Supplemental Table 1 details adjusted and unadjusted SHRs for each outcome. In the PIMT group, there were no significant differences in outcomes between the 42 patients with previously documented normal platelet counts and the 49 without them (supplemental Table 2).
Cumulative incidence of developing (A) any hematologic disease, (B) immune thrombocytopenia, and (C) hematologic neoplasia over up to 32 years of follow-up. Black hash symbols are censored events due to death or loss to follow-up. The 5-, 15-, and 25-year cumulative incidences with 95% confidence intervals for the persistent isolated mild thrombocytopenia group are additionally given.
Cumulative incidence of developing (A) any hematologic disease, (B) immune thrombocytopenia, and (C) hematologic neoplasia over up to 32 years of follow-up. Black hash symbols are censored events due to death or loss to follow-up. The 5-, 15-, and 25-year cumulative incidences with 95% confidence intervals for the persistent isolated mild thrombocytopenia group are additionally given.
Other outcomes of interest over the course of follow-up were also more frequent in the persistent mild thrombocytopenia group compared with the healthy subject group, including development of non-ITP systemic autoimmune disease (13% of the patients vs 3% of the patients), occurrence of clinically significant bleeding (24 patients [all in the setting of severe thrombocytopenia] vs 6 patients [all with normal platelet counts], and death [19/91 patients vs 20/364 patients]). Details regarding autoimmune diseases developed and bleeding events documented (according to International Society for Thrombosis and Haemostasis definitions) are listed in supplemental Table 3 and supplemental Table 4, respectively.
In this study, we describe long-term risk of developing hematologic disease in adults presenting with PIMT of unknown etiology. Controlling for age and the competing risk of death, we found a 19-fold higher risk of progression to diagnosis of ITP or hematologic neoplasia in these patients than in matched heathy subjects over an extended follow-up duration. A primary concern of thrombocytopenia, bleeding events, were ultimately observed in 26.4% of PIMT patients vs 1.7% of matched healthy subjects. These findings have important clinical implications in initial evaluation, counseling, surveillance, and follow-up of patients with PIMT. Our study expands considerably on prior work by Stasi and colleagues9 by describing both the incidence of ITP and hematologic neoplasia over a median follow-up nearly 4 times longer (20.5 years) and additionally evaluating risk in comparison to a matched healthy subject population in a rigorous statistical analysis. Our study has several strengths, namely careful development of the at-risk cohort, including thorough manual chart review by hematologists of decades of follow-up data, inclusion of a matched control group, and a long duration of consistent, continuous follow-up in both patient groups. It also has several important limitations, primarily the limited sample size (which limited the extent of covariate adjustment in regression models), retrospective data collection, and the requirement for baseline hematologist evaluation. This last limitation, albeit necessary to ensure that thrombocytopenia was truly of unknown etiology at time zero, could have imparted a potential selection bias onto our cohort, as the vast majority of patients initially identified by our database query did not have hematology evaluation.
In conclusion, adults with PIMT of unknown etiology are at high long-term risk for developing significant hematologic disease, a risk significantly higher than that in matched healthy subjects. Our findings suggest there may be value in regular (perhaps yearly) interval complete blood count follow-up of these patients, analogous to monoclonal gammopathy of undetermined significance (given comparable risks of progression). In addition, consideration of more systematic upfront workup, such as peripheral blood molecular testing for inherited and acquired myeloid mutations, may be of value. Directed study would certainly be required to evaluate and to confirm the optimal initial evaluation and longitudinal surveillance strategy in this patient population.
Acknowledgments
H.A.-S. is the recipient of the American Society of Hematology Scholar Award.
This work will be included in a dissertation submitted to the Faculty of Harvard Medical School in partial fulfillment of the requirements for the Degree of Master of Medical Sciences in Clinical Investigation (MMSCI) for N.A.
Authorship
Contribution: N.A. contributed to study design, data collection, data analysis, creation of tables and figures, writing of the first draft of the manuscript, critical revision of the manuscript, and final approval; R.F.G. and D.J.K. contributed to critical revision of the manuscript and final approval; and H.A.-S. contributed to study design, data collection, data analysis, creation of tables and figures, critical revision of the manuscript, and final approval.
Conflict-of-interest disclosure: None of the authors have disclosures relevant to the content of the manuscript. Universal disclosures include the following. D.J.K. has received research funding from Argenx, Biocryst, Immunovant, Rigel, Sanofi (Principia), Takeda (Bioverativ), and UCB; has been a consultant for Alexion (Syntimmune), Amgen, argenx, BioCryst, Bristol Myers Squibb, Caremark, Cellularity, Cellphire, CRICO, Daiichi Sankyo, Hengrui. Immunovant, Incyte, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Rigel, Sanofi (Bioveratif), Sanofi (Principia), Sanofi (Genzyme), Sobi (Dova), Takeda, UCB, and Up-To-Date; and owns stock in Rubius. R.F.G. has received research funding from Agios, Dova/Sobi, and Novartis; and has been a consultant for Agios and Principia/Sanofi. H.A.-S. has received research funding from Agios, Dova/Sobi, and Amgen; and been a consultant for Agios, Dova/Sobi, Forma, argenx, Rigel, Moderna, and Novartis. N.A. declares no competing financial interests.
Correspondence: Hanny Al-Samkari, Division of Hematology Oncology, Massachusetts General Hospital, Zero Emerson Pl, Suite 118, Office 112, Boston, MA 02114; e-mail: hal-samkari@mgh.harvard.edu.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal