In this issue of Blood, Melo-Cardenas et al explore the role of interleukin-13 (IL-13)/IL-4 signaling in myelofibrosis as an important pathway driving fibrotic progression through megakaryocyte expansion and increased transforming growth factor-β (TGF-β) production.1
“No mistakes in the tango, darling, not like life” proclaims the Al Pacino character in the tango scene from the 1992 movie, Scent of a Woman. To work, the tango requires both dance partners to move in relation to each other, a feat masterfully accomplished in this iconic movie scene. In myelofibrosis (MF), there are multiple different “tangos” at play in the bone marrow: hematopoietic-stromal, cell-cytokine, megakaryocyte-stem cell to name a few. The “tango” explored by Melo-Cardenas et al involves the cytokines, IL-13 and TGF-β, an interaction mediated via a key cellular driver of MF, megakaryocytes.
An array of cytokines, including IL-13, have been shown to be upregulated in MF.2 In addition, IL-13 has been implicated in solid organ fibrosis, such as in pulmonary fibrosis, through the activation of TGF-β1.3 Although TGF-β has been established as an important contributor to MF,4 the role of IL-13 on megakaryopoiesis and in MF has not been studied thus far. It is known, however, that in the context of interferon-driven inflammatory signaling, emergency megakaryopoiesis is activated through increased expression of megakaryocytic proteins in megakaryocyte-biased hematopoietic stem cells and progenitors.5 Although the JAK1/2 inhibitor ruxolitinib reduces multiple proinflammatory cytokines, resulting in symptomatic benefit for patients, not all inflammatory cytokines are effectively targeted by JAK pathway inhibition.6 In fact, in a preclinical mouse model of myeloproliferative neoplasm (MPN), IL-13 levels were not reduced following single-agent ruxolitinib treatment.7
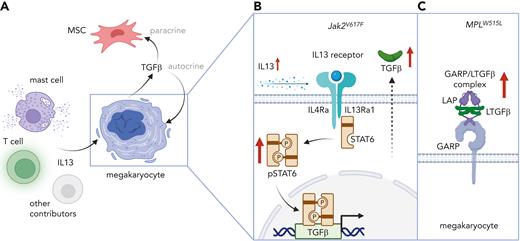
In their study, Melo-Cardenas et al first analyzed plasma and bone marrow supernatant from Jak2V617F and MPLW515L mice at both the prefibrotic and fibrotic stages of MPN disease. They found that multiple cytokines had increased levels, including IL-13, which was increased in the bone marrow supernatant in both models. They further showed that IL-13 promoted megakaryopoiesis in MPLW515L-expressing mice but not in wild-type animals (see figure). Within MPLW515L-expressing megakaryocytes stimulated by IL-13, they identified a significant increase in pathways involved in inflammatory responses and collagen biosynthesis, including in TGF-β signaling.
Melo-Cardenas and colleagues went on to verify increased IL-13 levels in the plasma of patients with MF. They found high expression of the IL-13 receptor α1 in megakaryocytes isolated from patients with MF compared with weak expression in megakaryocytes isolated from patients with essential thrombocythemia (ET) and polycythemia vera (PV). It is known that STAT6 signaling is activated downstream of the IL-13/IL-4 receptor and that this leads to the transcription of TGF-β. Concordant with this, they found increased level of phosphorylated STAT6 in megakaryocytes from both healthy donors and patients with MF following IL-13 stimulation (see figure).
To assess the effect of modulating IL-13 signaling in fibrosis progression, the authors investigated both IL-13 overexpression and IL-4ra knockout in MPN mouse models. Jak2V617F mice overexpressing IL-13 showed features of early bone marrow fibrosis with increased active TGF-β. Conversely, IL-4ra knockout reduced bone marrow fibrosis, decreased spleen weights in MPLW515L mice, and prolonged survival, findings with disease relevance for human MF. Last, Melo-Cardenas et al interrogated single-cell RNA-sequencing data from both prefibrotic and fibrotic bone marrow in both Jak2V617F and MPLW515L mice and found that mast cells and T cells were increased at the fibrotic stage, with both being known sources of IL-13.8 Concordant with this, bone marrow mast cells positive for TGF-β and IL-13 have been associated with higher fibrotic grade in human MF.9 A limitation of the study is that although the authors confirmed mast cells as a key cellular source of IL-13 in their MPN mouse models, they were not able to test if eradicating mast cells reduced the fibrotic phenotype. If that were the case, this would make mast cells an attractive cellular target, as suggested by prior studies in human MF.9
The translational potential of the study from Melo-Cardenas and colleagues, through targeting IL-13/IL-4 signaling in MF, is a strength of the article. Lebrikizumab, a monoclonal antibody directed against IL-13, in use for allergic diseases like asthma, is currently in phase 3 clinical trials.10 Dupilumab is a US Food and Drug Administration–approved monoclonal antibody against IL-4ra used for allergic diseases, like asthma and atopic dermatitis. Another clinically relevant aspect of the study is the fibrosis phenotype observed was TGF-β dependent. Given the historical challenges with therapeutically targeting TGF-β directly in patients with MF, indirect targeting via IL-13/IL-4 inhibition may be an alternative approach. A limitation of the study is that the authors were unable the test the efficacy of blocking IL-13/IL-4 signaling in a mouse model where MF is already established (eg, by using an antibody). Given the profound dysregulation of inflammatory cytokines in patients with MF, it is not clear how clinically impactful blocking a single pathway would be. Although the authors did not formally test this in their study, given the incomplete cytokine blockade achieved by JAK pathway inhibition in MF, a combinatorial clinical trial adding IL-13/IL-4 inhibition to a JAK inhibitor may be beneficial in patients.
In conclusion, in an excellent study with high translational relevance, Melo-Cardenas et al used a combination of mouse models, primary MPN samples, and single-cell approaches to uncover and validate a novel pathway in MF that can be therapeutically targeted. Through identifying and blocking key cytokine nodes in MF, it may be possible to “untangle” pathologic partnerships, such as IL-13–TGF-β, to slow down or even reverse bone marrow fibrosis.
IL-13 and TGF-β cooperate to promote myelofibrosis. (A) Mast cells, T cells, and potentially other cellular sources of IL-13 are expanded in the bone marrow of prefibrotic mice These cells release profibrotic IL-13, which stimulates the production of transforming growth factor-β (TGF-β) in megakaryocytes. MSC: mesenchymal stem cell. (B) In Jak2V617F mutant megakaryocytes carrying IL-13/IL-4 receptors, IL-13 leads to increased STAT6 phosphorylation (pSTAT6) signaling and increased TGF-β secretion. IL-4Ra: IL-4 receptor alpha. IL13Ra1: IL-13 receptor alpha 1. (C) MPLW515L mutant mice show increased levels of latency-associated peptide (LAP) and glycoprotein-A repetitions predominant protein (GARP), which binds latent TGF-β (LTGF-β) on the cell surface of megakaryocytes. Figure created with BioRender.com.
IL-13 and TGF-β cooperate to promote myelofibrosis. (A) Mast cells, T cells, and potentially other cellular sources of IL-13 are expanded in the bone marrow of prefibrotic mice These cells release profibrotic IL-13, which stimulates the production of transforming growth factor-β (TGF-β) in megakaryocytes. MSC: mesenchymal stem cell. (B) In Jak2V617F mutant megakaryocytes carrying IL-13/IL-4 receptors, IL-13 leads to increased STAT6 phosphorylation (pSTAT6) signaling and increased TGF-β secretion. IL-4Ra: IL-4 receptor alpha. IL13Ra1: IL-13 receptor alpha 1. (C) MPLW515L mutant mice show increased levels of latency-associated peptide (LAP) and glycoprotein-A repetitions predominant protein (GARP), which binds latent TGF-β (LTGF-β) on the cell surface of megakaryocytes. Figure created with BioRender.com.
Conflict-of-interest disclosure: A.M. receives research funding from Relay Therapeutics. A.M. has consulted for Janssen, PharmaEssentia, Actuate, Constellation, Aclaris, Cellarity, Morphic, BioMarin, Protagonist, and Incyte. A.M. has received research funding from Janssen and Actuate. J.S.J. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal