Key Points
CNB accurately diagnoses lymphoma in most instances but increases the risk of erroneous or nondefinitive conclusions.
Systematic expert review highly contributes to a precise lymphoma diagnosis, especially in cases sampled by CNB.
Abstract
According to expert guidelines, lymph node surgical excision is the standard of care for lymphoma diagnosis. However, core needle biopsy (CNB) has become widely accepted as part of the lymphoma diagnostic workup over the past decades. The aim of this study was to present the largest multicenter inventory of lymph nodes sampled either by CNB or surgical excision in patients with suspected lymphoma and to compare their diagnostic performance in routine pathologic practice. We reviewed 32 285 cases registered in the French Lymphopath network, which provides a systematic expert review of all lymphoma diagnoses in France, and evaluated the percentage of CNB and surgical excision cases accurately diagnosed according to the World Health Organization classification. Although CNB provided a definitive diagnosis in 92.3% and seemed to be a reliable method of investigation for most patients with suspected lymphoma, it remained less conclusive than surgical excision, which provided a definitive diagnosis in 98.1%. Discordance rates between referral and expert diagnoses were higher on CNB (23.1%) than on surgical excision (21.2%; P = .004), and referral pathologists provided more cases with unclassified lymphoma or equivocal lesion through CNB. In such cases, expert review improved the diagnostic workup by classifying ∼90% of cases, with higher efficacy on surgical excision (93.3%) than CNB (81.4%; P < 10−6). Moreover, diagnostic concordance for reactive lesions was higher on surgical excision than CNB (P = .009). Overall, although CNB accurately diagnoses lymphoma in most instances, it increases the risk of erroneous or nondefinitive conclusions. This large-scale survey also emphasizes the need for systematic expert review in cases of lymphoma suspicion, especially in those sampled by using CNB.
Introduction
Optimal management of patients with lymphoma depends on an accurate pathologic diagnosis, which is critical to guide the most appropriate therapy, especially in the era of individualized therapeutic approaches. However, diagnosing lymphoma remains challenging in some cases because it requires expertise and a large panel of ancillary tests.1-3 Technical advances in immunophenotyping and molecular techniques provide tools to improve the diagnosis of lymphomas and their classification among the >100 lymphoma subtypes recognized in the 2017 World Health Organization (WHO) classification.4
According to the European Society of Medical Oncology guidelines,5-7 surgical excision of lymphadenopathy is the gold standard when nodal lymphoma is suspected. Surgical excision allows a clear-cut analysis of the lymph node architecture and provides sufficient material for additional tests.8,9 However, over the past decades, minimally invasive methods such as core needle biopsy (CNB) have become widely accepted as part of the oncologic diagnostic workup and, according to the literature, may outperform surgical excision in the diagnosis of various lesions, including lymphomas.10 In fact, most published studies that compare the accuracy of CNB vs that of surgical excision for diagnosing lymphoma have shown that, diagnostically, both methods provide an equivalent performance.11-25 Compared with surgical excision, CNB is faster and easier to perform, presenting both lower risk of complications and lower cost.26 In addition, CNB can be performed in the elderly and in patients with comorbidities or when the sampling site is inaccessible to surgery.27-29 However, most of these studies were conducted with limited CNB cohorts of lymphoma patients at monocentric or regional levels,11-16,18-24,26,27,29-38 or mostly involved experienced radiologists in single institutions.13,16-23,27,32,34-37,39 In addition, the assessment of CNB accuracy may have been subject to some biases. For instance, some authors estimated the diagnostic performance of CNB by considering unclassified lymphomas as a definite diagnosis18,24 even though an accurate classification of lymphomas is required for optimal management. Other studies included a significant amount of relapsing lymphoma that strongly influenced diagnosis.11-18,21-23,26,32-34,38 Moreover, most studies excluded noninformative cases due to a lack of material, which is a major limitation of needle biopsy.10,12,13,15-18,21-24,26,27,31,35-38 Finally, the role of expert review in this setting has not been addressed. As a matter of fact, it is important to consider the risk of misdiagnosis especially on CNB because diagnosing lymphoma requires not only expertise but also a large panel of ancillary tests, which necessitates sufficient material.3,29
The current study presents a large inventory of lymph node CNB and surgical excision samples from patients with suspected lymphoma, from 2010 to 2018, recorded in the Lymphopath database, a national network aimed at providing an expert review of all lymphomas in France.3 The overall frequency and relative distribution of CNB and surgical excision sampling were analyzed. We then assessed the distribution of lymphoma subtypes according to both procedures and evaluated their diagnostic performances. Finally, the impact of expert review on diagnosing lymphoma when either CNB or surgical excision was performed is highlighted.
Methods
Patient selection and evaluation of sampling procedure distribution in the Lymphopath survey
Between January 2010 and December 2018, a total of 85 583 patients were registered in the French Lymphopath network aimed at providing an expert pathologic review of every newly diagnosed or suspected case of lymphoma before therapy in France. The Lymphopath review process has been previously published (by Laurent et al)3 and is briefly described in the supplemental Methods (available on the Blood website). Of note, relapse diagnoses may be submitted for expert review but are not recorded in the Lymphopath database. For each case, the following information was recorded from the Lymphopath network: patient’s age and sex, the biopsy site, the sampling procedure (CNB or surgical excision), the date of the biopsy, the private or public institution where the biopsy had been performed, the final diagnoses after expert review, and the initial diagnoses made by referral pathologists. Extranodal lymphomas such as primary cutaneous, digestive, or CNS lymphomas, as well as primary mediastinal B-cell lymphomas, were excluded because they are most commonly diagnosed on biopsy samples and hardly ever surgically removed. Then, after excluding cases in which the sampling procedure was not clearly reported, 32 285 lymph node samples (including 10 285 CNB and 22 000 surgical excision) were eligible for the study (supplemental Figure 1).
According to clinical practice in France,39 CNB procedures were almost always performed under imaging guidance (with the help of computed tomography imaging or ultrasound). The overall frequency of CNB and surgical excision in France and the distribution of lymphoma subtypes diagnosed by experts were analyzed according to the sampling procedure. Of note, 11 889 cases recorded from January 2010 to December 2013 (CNB, n = 3306; surgical excision, n = 8583) have already been reported in our previous study3 and 26% of cases (CNB, n = 2778; surgical excision, n = 5372) originated from reference centers. Data were collected anonymously, and the study was approved by the local ethics committee (Comité de Protection des Personnes Sud-Ouest et Outremer II).
Assessing the diagnostic performance of CNB and surgical excision after expert review
To evaluate the diagnostic performance of sampling procedures, expert diagnoses obtained by lymph node CNB (n = 10 285) and surgical excision (n = 22 000) were categorized into 3 groups: (1) cases with a definitive diagnosis corresponding to specific lymphoma subtypes of the WHO classification, neoplasms other than lymphomas, or benign lesions; (2) cases with a nondefinitive diagnosis corresponding to unclassified lymphomas or lymphomas with incomplete subtyping, or equivocal lesions that fall between reactive lesions and lymphoma; and (3) cases without diagnosis due to insufficient or inadequate material. We then evaluated the percentage of each category according to sampling procedures.
Evaluating the impact of an expert review on diagnosing lymphoma through CNB or surgical excision
As previously described, pathologists in France since 2010 have been encouraged to send every case of newly diagnosed or suspected lymphoma to a reference center in the Lymphopath pathology network before therapy.3 The Lymphopath database has recorded both the final diagnosis provided by the expert and the initial diagnosis proposed by the referral pathologist.
After excluding cases with no diagnosis proposed by the referral pathologist, 31 138 cases, including 9924 CNB and 21 214 surgical excision samples, were eligible for comparison of referral and expert diagnoses (supplemental Figure 1). To evaluate the potential impact of an expert review on CNB and surgical excision diagnoses, we first calculated the percentage of lymphomas ranked according to the WHO 2017 classification that did not change after review for both surgical excision and CNB sampling procedures. We then calculated the number of referral cases with nondefinitive diagnoses according to both CNB and surgical excision procedures (ie, unclassified lymphomas or equivocal diagnoses that fall between reactive lesions and lymphoma) that were subsequently accurately classified by experts.
To evaluate the potential impact of expert review on patients’ clinical management, misclassified lesions submitted by referral pathologists were divided into major and minor changes according to the guidelines of the National and European onco-hematology societies (Société Française d’Hématologie and European Society of Medical Oncology).3,40,41 For this analysis, only cases submitted by referral pathologists with classified diagnoses were selected (n = 26 117 [including n = 8026 CNBs and n = 18 091 surgical excisions]). The 5021 cases sent by referral pathologists with no definitive diagnosis were excluded (supplemental Figure 1).
Comparing diagnostic performance in paired CNB/surgical excision samples
To further evaluate CNB diagnostic performance compared with surgical excision, we analyzed cases in which both biopsy methods were performed. Within the whole cohort, 189 patients were identified who underwent CNB and subsequent surgical excision, both recorded into the Lymphopath database. It should be noted that this paired series is not exhaustive as all CNBs performed before surgical excision were not systematically recorded in the database. Once the cases with >3 months’ delay between the 2 biopsy procedures were excluded, 125 paired samples were identified in which surgical excision was performed because prior CNB had provided neither a definitive nor a confident diagnosis after expert review. We then evaluated diagnosis changes between CNB and paired surgical excision and assessed the impact of expert review on challenging cases that had needed additional surgical excision to either support or correct the suspected diagnosis.
Statistical analysis
Data are summarized by frequency and percentage for categorical variables and by median and range for continuous variables. Comparisons between surgical excision and CNB were assessed by using the χ2 test or Fisher’s exact test for qualitative variables and the Mann-Whitney U test for continuous variables. All statistical tests were two-sided, and P values <.05 were considered significant.
Results
Sampling procedure distribution in the Lymphopath survey
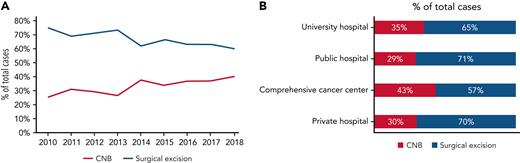
From January 2010 to December 2018, a total of 32 285 patients underwent either lymph node CNB (n = 10 285 [32%]) or surgical excision (n = 22 000 [68%]) for suspicion of lymphoma. The male-to-female ratio was 5.6:4.4, and patient ages ranged from 1 to 101 years (median age, 63 years). Patients were older in the CNB group than in the surgical excision group (patients aged >60 years, 62.3% vs 53%, respectively; P = .0001), and CNBs were more frequently performed in women (P = .0232). During this period, an increase in CNB procedures was observed, from 25% (n = 564 of 3393) to 40% (n = 1763 of 4396) of all lymph node biopsies in 2010 and 2018 (Figure 1A). Finally, the frequencies of CNB and surgical excision sampling were similar in both public (university and general hospital) and private institutions except for an increased proportion of CNB in comprehensive cancer centers compared with former institutions (P = .0001) (Figure 1B).
Overall frequency of sampling procedures for 32 285 patients with suspected lymphoma in France.
Overall frequency of sampling procedures for 32 285 patients with suspected lymphoma in France.
Expert diagnostic performance according to sampling procedures
After expert review, CNB and surgical excision provided a definitive diagnosis in 92.3% (n = 9497 of 10 285) and 98.1% (n = 21 584 of 22 000) of cases, respectively (P < .0001) (Table 1). Among the 10 285 CNB samples reviewed by experts, 85.4% corresponded to mature lymphomas ranked according to the WHO classification, 5.5% were reactive lesions, and 1.4% were nonlymphoid neoplasms. Among the 22 000 samples in the surgical excision cohort, 88% were diagnosed by experts as classified mature lymphomas, 8.6% were reactive lesions, and 1.5% were nonlymphoid neoplasms. Cases with nondefinitive diagnosis after expert review were more common on CNB than on surgical excision (5.9% and 1.8%; P < .0001). These cases comprised either unclassified lymphomas or equivocal diagnoses falling between reactive conditions and lymphomas; they were both more frequent on CNB (4.6% and 1.3%) compared with surgical excision (1.2% and 0.6%; P < .0001). In addition, CNB resulted in a higher number of cases with no diagnosis due to insufficient or inadequate material (1.8% and 0.1% with CNB and surgical excision; P < .0001).
Distribution of diagnoses after expert review according to sampling procedures in 32 285 patients with suspected lymphoma
| Expert diagnoses (n = 32 285) . | CNB (n = 10 285) . | Surgical excision (n = 22 000) . | P . |
|---|---|---|---|
| Definitive diagnosis | 9497 (92.3%) | 21 584 (98.1%) | <.0001 |
| Classified lymphoma | 8779 (85.4%) | 19 369 (88%) | |
| Nonlymphoid neoplasm | 148 (1.4%) | 327 (1.5%) | |
| Reactive lesion | 570 (5.5%) | 1888 (8.6%) | |
| Nondefinitive diagnosis | 606 (5.9%) | 403 (1.8%) | <.0001 |
| Unclassified lymphoma or lymphoma with incomplete subtyping | 468 (4.6%) | 277 (1.2%) | |
| Equivocal diagnosis between reactive lesion and lymphoma | 138 (1.3%) | 126 (0.6%) | |
| No diagnosis because of insufficient/inadequate material | 182 (1.8%) | 13 (0.1%) | <.0001 |
| Expert diagnoses (n = 32 285) . | CNB (n = 10 285) . | Surgical excision (n = 22 000) . | P . |
|---|---|---|---|
| Definitive diagnosis | 9497 (92.3%) | 21 584 (98.1%) | <.0001 |
| Classified lymphoma | 8779 (85.4%) | 19 369 (88%) | |
| Nonlymphoid neoplasm | 148 (1.4%) | 327 (1.5%) | |
| Reactive lesion | 570 (5.5%) | 1888 (8.6%) | |
| Nondefinitive diagnosis | 606 (5.9%) | 403 (1.8%) | <.0001 |
| Unclassified lymphoma or lymphoma with incomplete subtyping | 468 (4.6%) | 277 (1.2%) | |
| Equivocal diagnosis between reactive lesion and lymphoma | 138 (1.3%) | 126 (0.6%) | |
| No diagnosis because of insufficient/inadequate material | 182 (1.8%) | 13 (0.1%) | <.0001 |
Lymphoma distribution according to sampling procedures
The CNB and surgical excision cohorts showed the epidemiologic distribution of the 3 main categories of lymphomas triggering a majority of B-cell non-Hodgkin lymphomas (B-NHLs), followed by Hodgkin lymphomas (HLs) (including classical HL and nodular lymphocyte-predominant HL [NLPHL]) and peripheral T-cell lymphomas (PTCLs). However, when we compared lymphoma subtype frequencies according to sampling procedures, B-NHL diagnoses were more frequent in the CNB cohort than in the surgical excision cohort (74.9% vs 65.9% of lymphoma diagnoses, respectively; P < .0001). In contrast, diagnoses of HL and PTCL were more common on surgical excision than CNB (25.6% vs 18.7% of HL and 8.5% vs 6.4% of PTCL; P < .0001) (Table 2). The increase of B-NHL diagnoses in the CNB cohort was mainly due to the diffuse large B-cell lymphoma, not otherwise specified (DLBCL NOS) subtype representing 42.4% of B-NHL vs 32.3% in the surgical excision cohort (P < .0001) (Figure 2A). Moreover, the most aggressive B-NHLs such as Burkitt lymphoma and high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements were also more frequently diagnosed through CNB than surgical excision (1.2% and 1.6% of CNB B-NHL cases vs 0.8% and 0.8% of surgical excision B-NHL cases; P = .0031 and P < .0001). Conversely, small B-cell lymphomas, including follicular lymphoma (grades 1, 2, and 3A), chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma, and nodal marginal zone lymphoma (NMZL), were more frequent in the surgical excision cohort (P < .001, P < .0001, P < .01, and P < .01).
Repartition of the 28 148 classified lymphomas after pathologic review, according to sampling procedures
| Expert diagnoses (n = 28 148) . | CNB (n = 8779) . | Surgical excision (n = 19 369) . | P . |
|---|---|---|---|
| Classified B-cell lymphoma (n = 19 347) | 6574 (74.9%) | 12 773 (65.9%) | <.0001 |
| Aggressive B-NHL | 3090 (35.2%) | 4662 (24.1%) | |
| Indolent B-NHL | 3484 (39.7%) | 8111 (41.9%) | |
| Classified peripheral T-cell lymphoma (n = 2201) | 565 (6.4%) | 1636 (8.5%) | <.0001 |
| Classified HL (n = 6600) | 1640 (18.7%) | 4960 (25.6%) | <.0001 |
| Classical HL | 1511 (17.2%) | 4297 (22.2%) | |
| Nodular lymphocyte predominant HL | 129 (1.5%) | 663 (3.4%) |
| Expert diagnoses (n = 28 148) . | CNB (n = 8779) . | Surgical excision (n = 19 369) . | P . |
|---|---|---|---|
| Classified B-cell lymphoma (n = 19 347) | 6574 (74.9%) | 12 773 (65.9%) | <.0001 |
| Aggressive B-NHL | 3090 (35.2%) | 4662 (24.1%) | |
| Indolent B-NHL | 3484 (39.7%) | 8111 (41.9%) | |
| Classified peripheral T-cell lymphoma (n = 2201) | 565 (6.4%) | 1636 (8.5%) | <.0001 |
| Classified HL (n = 6600) | 1640 (18.7%) | 4960 (25.6%) | <.0001 |
| Classical HL | 1511 (17.2%) | 4297 (22.2%) | |
| Nodular lymphocyte predominant HL | 129 (1.5%) | 663 (3.4%) |
Overall frequency of NHL subtypes after expert review, according to sampling procedures (n = 21 548). ALCL, anaplastic large cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL1,2,3A, grades 1, 2, and 3A follicular lymphoma; FL3B, grade 3B follicular lymphoma; HGBL, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements; LPL/WD, lymphoplasmacytic lymphoma/Waldenström macroglobulinemia; MCL, mantle cell lymphoma; ns, not significant; PTCL NOS, PTCL, not otherwise specified; TCRBCL, T-cell/histiocyte-rich B-cell lymphoma. Significance levels: ns, P > .05; ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
Overall frequency of NHL subtypes after expert review, according to sampling procedures (n = 21 548). ALCL, anaplastic large cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL1,2,3A, grades 1, 2, and 3A follicular lymphoma; FL3B, grade 3B follicular lymphoma; HGBL, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements; LPL/WD, lymphoplasmacytic lymphoma/Waldenström macroglobulinemia; MCL, mantle cell lymphoma; ns, not significant; PTCL NOS, PTCL, not otherwise specified; TCRBCL, T-cell/histiocyte-rich B-cell lymphoma. Significance levels: ns, P > .05; ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
The increased proportion of PTCL diagnoses in the surgical excision cohort compared with the CNB cohort was related to a higher number of angioimmunoblastic T-cell lymphomas (AITLs) (56.2% and 44.1% of PTCL, respectively; P < .0001) (Figure 2B). In contrast, other PTCLs such as anaplastic lymphoma kinase–positive (ALK+) or –negative (ALK–) anaplastic large cell lymphomas were more frequently observed in the CNB cohort than in the surgical excision cohort (P < .0001 and P = .01). The frequency of PTCL NOS showed no significant difference between CNB and surgical excision (28.5% vs 26.4%). Regarding HL, the diagnosis of NLPHL was significantly more frequent on surgical excision (P < .0001) (Table 2).
Overall concordance between referral and expert pathologist diagnoses according to sampling procedures
After excluding cases referred without diagnoses, 31 138 cases (including 9924 CNB and 21 214 surgical excision lymph node samples) were eligible to compare referral vs expert diagnoses (supplemental Figures 1 and 2). The overall concordance rate between referral and expert pathologists’ diagnoses after Lymphopath review was 78.2% (n = 24 339 of 31 138). This overall concordance rate was higher in the surgical excision cohort (78.8%; n = 16 712 of 21 214) compared with the CNB cohort (76.9%; n = 7627 of 9924; P = .004). It is worth noting that among the lymphoma cases properly ranked according to the WHO classification, the rates of concordant diagnoses between referral and expert pathologists were similar for CNB and surgical excision sampling up to 80.5% (n = 6844 of 8499 accurately classified lymphomas) and 80.4% (n = 15 086 of 18 760), respectively. Nevertheless, concordance rates varied according to lymphoma subtypes, from 29.5% to 89.9% on CNB and from 44.7% to 93.9% on surgical excision (Table 3). Among B-NHL cases, the concordance rate was high and similar on CNB and surgical excision (80.2% and 79.2%). For instance, the DLBCL NOS concordance rate reached 86% and 82.2% on CNB and surgical excision procedures, followed by Burkitt lymphoma (76.6% and 81.3%) and T-cell/histiocyte-rich large B-cell lymphomas (72.7% and 69%). Misdiagnoses of grade 3B follicular lymphoma and high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements were the most frequent in both cohorts, with concordance rates of 29.5% and 40.2% on CNB, vs 44.8% and 44.7% on surgical excision. Nevertheless, we observed a lower concordance for grade 3B follicular lymphoma diagnosis on CNB than on surgical excision (P = .032). Regarding small B-cell lymphomas, concordance rates between expert and referral diagnoses were high with both CNB and surgical excision procedures for mantle cell lymphoma (83.4% vs 80.4%), chronic lymphocytic leukemia/small lymphocytic lymphoma (83.2% vs 79.8%), and follicular lymphoma grades 1, 2, and 3A (78.1% vs 82.6%). However, the latter follicular lymphoma cases were more frequently diagnosed by the referral pathologist through surgical excision (P < .0001). Diagnostic concordance decreased in more challenging small B-cell lymphoma subtypes such as NMZL and lymphoplasmacytic lymphoma/Waldenström disease with both CNB and surgical excision procedures. The concordance was unexpectedly higher on CNB regarding lymphoplasmacytic lymphoma/Waldenström disease diagnosis within the limits of the current series (P = .012), however.
Concordance rates between referral and expert diagnoses according to sampling procedures in 31 138 eligible cases
| Lymphoma diagnoses . | CNB concordance . | Surgical excision concordance . |
|---|---|---|
| Overall concordance | 76.9% (n = 7627/9924) | 78.8% (n = 16 712/21 214)∗∗ |
| Classified lymphoma | 80.5% (n = 6844/8499) | 80.4% (n = 15 086/18 760) |
| Classified B-NHL | 80.2% (n = 5110/6368) | 79.2% (n = 9787/12 359) |
| DLBCL NOS | 86% (n = 2319/2698) | 82.2% (n = 3290/4001)∗∗∗ |
| TCRBCL | 72.7% (n = 40/55) | 69% (n = 78/113) |
| HGBL | 40.2% (n = 41/102) | 44.7% (n = 42/94) |
| BL | 76.6% (n = 59/77) | 81.3% (n = 78/96) |
| Grade 3B FL | 29.5% (n = 18/61) | 44.8% (n = 95/212)∗ |
| Grades 1, 2, and 3A FL | 78.1% (n = 1665/2133) | 82.6% (n = 3697/4475)∗∗∗ |
| NMZL | 63.4% (n = 192/303) | 56.8% (n = 389/685) |
| CLL/SLL | 83.2% (n = 381/458) | 79.8% (n = 1267/1587) |
| MCL | 83.4% (n = 331/397) | 80.4% (n = 761/946) |
| LPL/WD | 76.2% (n = 64/84) | 60% (n = 90/150)∗ |
| Classified PTCL | 63.5% (n = 345/543) | 62.3% (n = 980/1572) |
| PTCL NOS | 61% (n = 94/154) | 54.4% (n = 228/419) |
| AITL | 58.4% (n = 142/243) | 64.6% (n = 565/874) |
| ALCL ALK– | 68.1% (n = 49/72) | 58.0% (n = 87/150) |
| ALCL ALK+ | 81.1% ( = 60/74) | 77.5% (n = 100/129) |
| Classified HL | 87.5% (n = 1389/1588) | 89.4% (n = 4319/4829)∗ |
| cHL | 89.9% (n = 1318/1466) | 93.9% (n = 3944/4201)∗∗∗ |
| NLPHL | 58.2% (n = 71/122) | 59.7% (n = 375/628) |
| Nonlymphoid neoplasm | 65.5% (n = 93/142) | 63.6% (n = 196/308) |
| Reactive lesion | 63.6% (n = 337/530) | 69.6% (n = 1222/1756)∗∗ |
| Lymphoma diagnoses . | CNB concordance . | Surgical excision concordance . |
|---|---|---|
| Overall concordance | 76.9% (n = 7627/9924) | 78.8% (n = 16 712/21 214)∗∗ |
| Classified lymphoma | 80.5% (n = 6844/8499) | 80.4% (n = 15 086/18 760) |
| Classified B-NHL | 80.2% (n = 5110/6368) | 79.2% (n = 9787/12 359) |
| DLBCL NOS | 86% (n = 2319/2698) | 82.2% (n = 3290/4001)∗∗∗ |
| TCRBCL | 72.7% (n = 40/55) | 69% (n = 78/113) |
| HGBL | 40.2% (n = 41/102) | 44.7% (n = 42/94) |
| BL | 76.6% (n = 59/77) | 81.3% (n = 78/96) |
| Grade 3B FL | 29.5% (n = 18/61) | 44.8% (n = 95/212)∗ |
| Grades 1, 2, and 3A FL | 78.1% (n = 1665/2133) | 82.6% (n = 3697/4475)∗∗∗ |
| NMZL | 63.4% (n = 192/303) | 56.8% (n = 389/685) |
| CLL/SLL | 83.2% (n = 381/458) | 79.8% (n = 1267/1587) |
| MCL | 83.4% (n = 331/397) | 80.4% (n = 761/946) |
| LPL/WD | 76.2% (n = 64/84) | 60% (n = 90/150)∗ |
| Classified PTCL | 63.5% (n = 345/543) | 62.3% (n = 980/1572) |
| PTCL NOS | 61% (n = 94/154) | 54.4% (n = 228/419) |
| AITL | 58.4% (n = 142/243) | 64.6% (n = 565/874) |
| ALCL ALK– | 68.1% (n = 49/72) | 58.0% (n = 87/150) |
| ALCL ALK+ | 81.1% ( = 60/74) | 77.5% (n = 100/129) |
| Classified HL | 87.5% (n = 1389/1588) | 89.4% (n = 4319/4829)∗ |
| cHL | 89.9% (n = 1318/1466) | 93.9% (n = 3944/4201)∗∗∗ |
| NLPHL | 58.2% (n = 71/122) | 59.7% (n = 375/628) |
| Nonlymphoid neoplasm | 65.5% (n = 93/142) | 63.6% (n = 196/308) |
| Reactive lesion | 63.6% (n = 337/530) | 69.6% (n = 1222/1756)∗∗ |
ALCL, anaplastic large cell lymphoma; BL, Burkitt lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FL, follicular lymphoma; HGBL, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements; cHL, classical HL; LPL/WD, lymphoplasmacytic lymphoma/Waldenström macroglobulinemia; MCL, mantle cell lymphoma; PTCL NOS, PTCL, not otherwise specified; TCRBCL, T-cell/histiocyte-rich B-cell lymphoma.
P value significance levels: ∗≤.05; ∗∗≤.01; ∗∗∗≤.0001.
The concordance rates for PTCL diagnoses were lower than for B-NHL subtypes: ∼60% in both CNB and surgical excision cohorts. The percentages of misdiagnoses according to PTCL subtypes were similar with both procedures, varying from 58.4% to 81.1% on CNB and from 54.4% to 77.5% on surgical excision, with the highest concordance for anaplastic large cell lymphoma ALK+.
Finally, with respect to HL, both procedures yielded similar concordance rates between referral and expert pathologists: ∼90% of diagnostic agreement in both cohorts. However, classical HL was more often accurately diagnosed by referral pathologists on surgical excision (93.9%) than on CNB (89.9%; P < .0001). NLPHL diagnosis showed no significant difference in concordance rate between CNB and surgical excision sampling (∼60% of concordance). Interestingly, reactive lesions were better diagnosed by referral on surgical excision (69.6%) than on CNB (63.5%; P = .009).
Impact of expert pathologic review according to sampling procedures
Among the 31 138 eligible cases, 6799 (21.8%) were provided with discordant diagnoses between referral and expert pathologists, including 2339 misclassified lesions (7.5%) and 4460 nondefinitive diagnoses (14.3%) that were further changed by experts (supplemental Figure 1). Concordant diagnoses encompassed 23 778 referral classified lesions confirmed by the experts and 561 nondefinitive diagnoses submitted by referral pathologists without modification after expert review. After excluding referral cases submitted without a definitive (ie, classified) diagnosis (n = 5021), CNB and surgical excision referral misdiagnoses were evaluated according to their predicted impact on patients’ care. Major changes represented 8% of CNB (n = 646 of 8026) and 7.6% of surgical excision (n = 1371 of 18 091) cases. They comprised lymphoma subtype misclassifications (n = 472 CNB and n = 1130 surgical excision), changes between lymphomas and nonlymphoid neoplasms (n = 28 and n = 50, respectively), and changes between lymphomas or nonlymphoid neoplasms and reactive lesions (n = 146 and n = 191). Minor changes represented 1.3% of CNB cases and 1.2% of surgical excision cases (n = 106 of 8026 and 216 of 18 091), including changes between grade 3B follicular lymphoma and DLBCL NOS, and reclassifications of DLBCL or PTCL subtypes without therapeutic impact (Table 4).
Impact of expert review on 26 117 referral classified diagnoses according to sampling procedures
| Diagnostic changes . | CNB (n = 8026) . | Surgical excision (n = 18 091) . | P . |
|---|---|---|---|
| Discordant cases (n = 2339) | 752 (9.3%) | 1587 (8.7%) | .12 |
| Major changes | 646 (8.0%) | 1371 (7.6%) | .40 |
| Misclassifications of lymphoma subtypes | 472 (5.9%) | 1130 (6.2%) | .26 |
| B-NHL vs PTCL∗ | 29 (0.3%) | 76 (0.4%) | |
| cHL vs B-NHL† | 39 (0.5%) | 96 (0.5%) | |
| cHL vs PTCL‡ | 34 (0.4%) | 90 (0.5%) | |
| NLPHL vs other lymphoma subtypes§ | 32 (0.4%) | 160 (0.8%) | |
| “High-grade” vs “low-grade” B-NHLǁ | 153 (1.9%) | 344 (1.9%) | |
| Misclassifications in “low-grade” B-NHL with therapeutic impact¶ | 119 (1.5%) | 260 (1.4%) | |
| Misclassifications in “high-grade” B-NHL with therapeutic impact# | 65 (0.7%) | 96 (0.5%) | |
| Misclassifications in classified PCTL with therapeutic impact∗∗ | 1 (0.01%) | 8 (0.04%) | |
| Misclassifications between lymphomas and other neoplasms | 28 (0.3%) | 50 (0.3%) | .32 |
| From classified lymphomas to nonlymphoid neoplasms | 15 (0.2%) | 26 (0.2%) | |
| From nonlymphoid neoplasms to classified lymphomas | 13 (0.1%) | 24 (0.1%) | |
| Misclassifications between malignant and reactive lesions | 146 (1.8%) | 191 (1%) | <.00001 |
| From classified lymphomas or nonlymphoid neoplasms to reactive lesions | 89 (1.1%) | 90 (0.5%) | |
| From reactive lesions to classified lymphomas or nonlymphoid neoplasms | 57 (0.7%) | 101 (0.5%) | |
| Minor changes | 106 (1.3%) | 216 (1.2%) | .39 |
| From DLBCL subtypes to other DLBCL subtypes without therapeutic impact†† | 56 (0.7%) | 57 (0.3%) | |
| DLBCL vs grade 3B FL | 22 (0.3%) | 51 (0.3%) | |
| From PTCL to other PCTL subtypes without therapeutic impacts‡‡ | 28 (0.4%) | 108 (0.6%) | |
| Concordant cases (n = 23 778) | 7274 (90.7%) | 16 504 (91.3%) | .12 |
| Diagnostic changes . | CNB (n = 8026) . | Surgical excision (n = 18 091) . | P . |
|---|---|---|---|
| Discordant cases (n = 2339) | 752 (9.3%) | 1587 (8.7%) | .12 |
| Major changes | 646 (8.0%) | 1371 (7.6%) | .40 |
| Misclassifications of lymphoma subtypes | 472 (5.9%) | 1130 (6.2%) | .26 |
| B-NHL vs PTCL∗ | 29 (0.3%) | 76 (0.4%) | |
| cHL vs B-NHL† | 39 (0.5%) | 96 (0.5%) | |
| cHL vs PTCL‡ | 34 (0.4%) | 90 (0.5%) | |
| NLPHL vs other lymphoma subtypes§ | 32 (0.4%) | 160 (0.8%) | |
| “High-grade” vs “low-grade” B-NHLǁ | 153 (1.9%) | 344 (1.9%) | |
| Misclassifications in “low-grade” B-NHL with therapeutic impact¶ | 119 (1.5%) | 260 (1.4%) | |
| Misclassifications in “high-grade” B-NHL with therapeutic impact# | 65 (0.7%) | 96 (0.5%) | |
| Misclassifications in classified PCTL with therapeutic impact∗∗ | 1 (0.01%) | 8 (0.04%) | |
| Misclassifications between lymphomas and other neoplasms | 28 (0.3%) | 50 (0.3%) | .32 |
| From classified lymphomas to nonlymphoid neoplasms | 15 (0.2%) | 26 (0.2%) | |
| From nonlymphoid neoplasms to classified lymphomas | 13 (0.1%) | 24 (0.1%) | |
| Misclassifications between malignant and reactive lesions | 146 (1.8%) | 191 (1%) | <.00001 |
| From classified lymphomas or nonlymphoid neoplasms to reactive lesions | 89 (1.1%) | 90 (0.5%) | |
| From reactive lesions to classified lymphomas or nonlymphoid neoplasms | 57 (0.7%) | 101 (0.5%) | |
| Minor changes | 106 (1.3%) | 216 (1.2%) | .39 |
| From DLBCL subtypes to other DLBCL subtypes without therapeutic impact†† | 56 (0.7%) | 57 (0.3%) | |
| DLBCL vs grade 3B FL | 22 (0.3%) | 51 (0.3%) | |
| From PTCL to other PCTL subtypes without therapeutic impacts‡‡ | 28 (0.4%) | 108 (0.6%) | |
| Concordant cases (n = 23 778) | 7274 (90.7%) | 16 504 (91.3%) | .12 |
The 5021 cases submitted by referral pathologists with no definitive diagnosis were excluded. cHL, classical HL; FL, follicular lymphoma.
Twelve CNB and 42 surgical excision cases initially referred as classified B-NHL were modified to classified PTCL, and 17 CNB and 34 surgical excision cases referred as classified PTCL were modified to classified B-NHL.
Twenty-five CNB and 76 surgical excision cases initially diagnosed as cHL were modified to classified B-NHL, 14 CNB and 20 surgical excision cases initially referred as classified B-NHL were modified to cHL.
Thirteen CNB and 64 surgical excision cases initially diagnosed as cHL were modified to classified PTCL, 21 CNB and 26 surgical excision cases initially referred as classified PTCL were modified to cHL.
Fourteen CNB and 59 surgical excision cases initially diagnosed as NLPHL were modified to other classified lymphomas, 18 CNB and 101 surgical excision cases classified as lymphomas other than NLPHL were modified to NLPHL.
Eighty CNB cases referred as “high-grade” B-NHL (including 53 DLBCL NOS, 4 T-cell/histiocyte-rich B-cell lymphomas [TCRBCL], and 23 grade 3B FL) were modified to 4 chronic lymphocytic leukemias (CLL), 65 FL, 3 lymphoplasmacytic lymphomas/Waldenström macroglobulinemia (LPL/WD), 2 mantle cell lymphomas (MCL), and 6 NMZLs. A total of 204 surgical excision cases referred as “high-grade” B-NHL were modified to 31 CLL; 111 grades 1, 2, and 3A FL; 1 LPL/WD; 35 MCL; and 26 NMZL. Seventy-three CNB referred as “low-grade” B-NHL (including 3 CLL; 47 grades 1, 2, and 3A FL; 1 LPL/WD; 17 MCL; and 5 NMZL) were modified to classified “high-grade” B-NHL (including 55 DLBCL NOS, 1 TCRBCL, 1 Burkitt lymphoma [BL], 2 high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements [HGBL], and 14 grade 3B FL). A total of 140 surgical excision referred as “low-grade” B-NHL (including 6 CLL; 105 grades 1, 2, and 3A FL; 6 LPL; 20 MCL; and 3 NMZL) were modified to classified “high-grade” B-NHL (107 DLBCL NOS, 2 HGBL, and 31 grade 3B FL with surgical excision).
A total of 119 CNB (including 34 CLL; 45 grades 1, 2, and 3A FL; 6 LPL/WD; 10 MCL; and 24 NMZL) and 260 surgical excision (45 CLL; 92 grades 1, 2, and 3A FL; 21 LPL/WD; 48 MCL; and 54 NMZL) were misclassifications between “low-grade” B-NHLs with a potential therapeutic impact.
Sixty-five CNB and 96 surgical excision cases were misclassifications between DLBCL NOS and HGBL (40 CNB and 66 surgical excision), misclassifications between BL and HGBL (14 CNB and 22 surgical excision), and misclassifications between BL and HGBL (11 CNB and 8 surgical excision cases).
One CNB case and 8 surgical excision cases were misclassifications between anaplastic large cell lymphomas (ALCL) ALK+ or ALK– and other PTCL.
Fifty-six CNB and 57 surgical excision cases were DLBCL subtype misclassifications without potential impact on patients’ care and included changes between DLBCL NOS, TCRBCL, DLBCL Epstein-Barr virus positive, and DLBCL ALK+.
Twenty-eight CNB (6 AITL, 12 PTCL NOS, and 10 ALCL ALK–) and 108 surgical excision (44 AITL, 44 PTCL, and 20 ALCL ALK–) were PTCL subtype misclassifications without potential impact on patients’ care.
Regarding nondefinitive diagnoses, as expected, they were more frequently made by referral pathologists than experts regardless of sampling procedure, representing 16.1% and 3.7% of the whole cohort, respectively (P < .0001). However, CNB provided more referral nondefinitive diagnoses than surgical excision (19.1% vs 14.7%; P < .00001). In this setting, expert review eventually reached definitive diagnoses in 88.8% of cases (n = 4460 of 5021). Nevertheless, the diagnostic performance of expert pathologic review remained higher on surgical excision, with a change from nondefinitive to complete diagnoses in 93.3% of cases compared with 81.4% on CNB (P < .00001) (Table 5).
Impact of expert review on 5021 referral nondefinitive diagnoses according to sampling procedures
| Diagnostic changes . | CNB (n = 1898) . | Surgical excision (n = 3123) . | P . |
|---|---|---|---|
| Changes to definite diagnoses (n = 4460) | 1545 (81.4%) | 2915 (93.3%) | <.00001 |
| Unclassified lymphoma to classified lymphoma | 925 (48.7%) | 1707 (54.7%) | |
| Unclassified lymphoma to nonlymphoid neoplasm or reactive lesion | 29 (1.5%) | 91 (2.9%) | |
| Uncertain to lymphoma | 418 (22.0%) | 635 (20.3%) | |
| Uncertain to nonlymphoid neoplasm or reactive lesion | 173 (9.1%) | 482 (15.4%) | |
| No changes (n = 561) | 353 (18.6%) | 208 (6.7%) | <.00001 |
| Diagnostic changes . | CNB (n = 1898) . | Surgical excision (n = 3123) . | P . |
|---|---|---|---|
| Changes to definite diagnoses (n = 4460) | 1545 (81.4%) | 2915 (93.3%) | <.00001 |
| Unclassified lymphoma to classified lymphoma | 925 (48.7%) | 1707 (54.7%) | |
| Unclassified lymphoma to nonlymphoid neoplasm or reactive lesion | 29 (1.5%) | 91 (2.9%) | |
| Uncertain to lymphoma | 418 (22.0%) | 635 (20.3%) | |
| Uncertain to nonlymphoid neoplasm or reactive lesion | 173 (9.1%) | 482 (15.4%) | |
| No changes (n = 561) | 353 (18.6%) | 208 (6.7%) | <.00001 |
Comparison of diagnostic performance in paired CNB/surgical excision samples
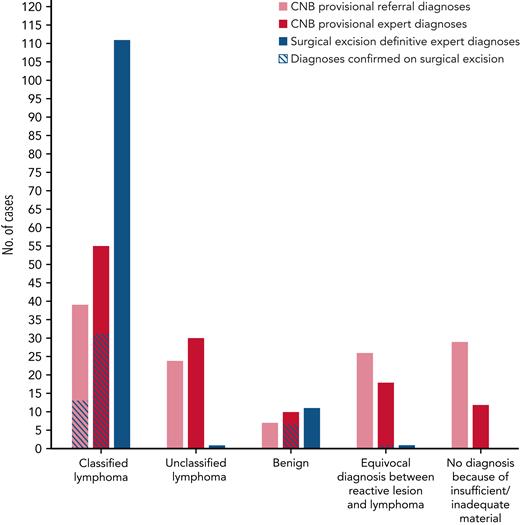
Within the whole cohort, 125 patients underwent both CNB and surgical excision sequentially performed due to nondefinitive diagnosis after expert review on CNB. The median interval time between CNB and subsequent surgical excision was 31 days (range, 3-92 days). When anatomic site was available (90% of cases; n = 112 of 125), lymph nodes were predominantly axillar (n = 34 [27.2%]), followed by groin (n = 31 [24.8%]) and superficial cervical lymph nodes (n = 28 [22.4%]). Deep lymph node biopsies (eg, abdominal) represented 15.2% (n = 19) of biopsied sites. Among them, initial diagnoses suspected on CNB were confirmed on 21.5% of subsequent surgical excision cases, representing 23.6% of superficial samples and 10.5% of deep lymph node samples (n = 22 of 93 and n = 2 of 19, respectively). There were no statistically significant differences in discordance rates between anatomic sites (P = .2). In contrast to surgical excision samples, CNB samples were characterized by a high number of nondefinitive diagnoses such as: (1) unclassified lymphomas (representing 19.2% and 24% of referral and expert CNB diagnoses); (2) uncertain between reactive and lymphoma lesions (20.8% and 14.4%); and (3) cases with no diagnosis because of insufficient or inadequate material (23.2% and 9.6% of referral and expert CNB diagnoses) (supplemental Table 1). The remaining cases were suspicion of classified lymphomas (31.2% and 44% of referral and expert CNB diagnoses) or reactive lesions (5.6% and 8%). Lymphoma suspicions tended to be more frequently confirmed on subsequent surgical excision when CNB had been assessed by experts (56.40% vs 33.3% of expert and referral diagnoses confirmed on surgical excision) (supplemental Table 2). Likewise, most of the reactive lesions suspected by experts on CNB were confirmed on surgical excision (n = 7 of 11), whereas all referral reactive lesions were reclassified as lymphoma on surgical excision (n = 7) (Figure 3; supplemental Table 2).
Comparison of CNB provisional and surgical excision definitive diagnoses made on 125 paired CNB/surgical excision samples.
Comparison of CNB provisional and surgical excision definitive diagnoses made on 125 paired CNB/surgical excision samples.
Among discordant CNB/surgical excision expert diagnoses, most discrepancies concerned PTCL, especially including AITL, as none of them (n = 10) had been correctly diagnosed on CNB. Other main discrepancies included NMZL (n = 1 of 8 cases accurately diagnosed on CNB), NLPHL (n = 2 of 11), and HL (n = 9 of 25) (supplemental Table 2). We also observed several misdiagnoses between NLPHL and T-cell/histiocyte-rich B-cell lymphoma (n = 3) and also between AITL and HL (n = 6). Interestingly, 18.2% (n = 4 of 22) of DLBCL cases diagnosed on surgical excision had been erroneously classified as low-grade B-cell lymphomas.
Discussion
Based on this largest multicenter survey to date, within the Lymphopath network, CNB seems to be a reliable diagnostic approach for patients with suspected lymphoma, providing a definitive diagnosis in 92.3% of cases after expert review. The high diagnostic accuracy of CNB in diagnosing lymphoma has been previously reported in the literature.11-39,42 However, most of these studies did not assess the percentage of CNB cases accurately ranked according to the WHO classification criteria.4 This issue constitutes an important limitation in the assessment of diagnostic accuracy and raises major concerns regarding provision of the best therapeutic management.15,23,25 Moreover, these studies assessed CNB diagnostic performance in smaller cohorts at monocentric or regional levels.5-7 In our large study, we highlight an important limitation of CNB in the clinical setting: significantly more patients are provided with nondefinitive diagnoses and insufficient/inadequate material through CNB (7.7%) than the gold standard surgical excision (1.9%). We also emphasize the need for expert review,3 especially in the context of CNB, by illustrating the difficulties of histologic interpretation on small biopsy specimens that require more skills and experience to accurately diagnose lymphoma after a selective choice of ancillary tests.
Among classified lymphomas, CNB displayed the same epidemiologic distribution of the main lymphoma categories as surgical excision, although some discrepancies were observed in the distribution of several subtypes according to sampling procedures. Notably, aggressive B-NHLs, unlike indolent B-NHLs, were more frequently diagnosed on CNB than on surgical excision. As a matter of fact, high-grade B-NHLs are clinically aggressive and require urgent therapeutic management, which can be achieved faster with CNB than with surgical excision. In contrast, HLs and PTCLs were more frequently diagnosed on surgical excision. Such diagnoses have previously appeared to be challenging on CNB,12,17,18,23 which might explain the predilection for surgical excision sampling in cases of suspected HL or PTCL. In fact, samples with limited material may lead to misdiagnosis of some lymphoma subtypes, mainly including those that contain few tumor cells in a polymorphic background such as HL and/or those that exhibit heterogeneous distribution or specific microenvironmental features such as AITL. As highlighted by our paired CNB/surgical excision cohort, AITL diagnosis may be challenging on CNB because it requires careful assessment of the lymph node architecture to meet diagnosis criteria such as an expanded meshwork of follicular dendritic cells and prominent high endothelial venules.43 Moreover, large atypical CD30+ cells appearing in the AITL background may closely resemble Hodgkin or Reed-Sternberg cells and thus lead to an erroneous HL diagnosis. These results emphasize the difficulty in making a definitive diagnosis of AITL or HL on CNB and highly encourage performing additional surgical excision in doubtful cases, even in the setting of expert review.
Of note, diagnoses of unclassified lymphoma or uncertain lesions falling between reactive process and lymphoma significantly increase in cases assessed by nonexpert pathologists, especially on CNB compared with surgical excision. In such cases, expert review improves the final diagnosis in most instances by applying the WHO classification criteria, even though this expertise remains more efficient on surgical excision than on CNB. Although some referral misdiagnoses may be due to limited access to advanced techniques such as molecular tests, it should be noted that referral pathologists nowadays have access to extensive panels of antibodies in their own laboratories, suitable to diagnose the majority of lymphoma subtypes.3 With respect to erroneously classified lesions made by referral pathologists, changes from reactive lesions to lymphoma or nondefinitive diagnoses after expert review were higher on CNB, resulting in a critical change in the clinical management of patients. These findings highlight the main CNB limitations that may require a second assessment through surgical excision, resulting in treatment delays with a potential impact on patients’ survival. As previously reported, the accuracy of CNB diagnosis might be improved by performing additional fine needle aspiration with ancillary techniques.38 However, lymph node immunophenotyping by flow cytometry is not routinely performed in France and requires dedicated fresh material only rarely available in the context of this national network. Finally, CNB diagnostic performance may be improved by increasing the needle gauge and the number of cores.13,22,36,44 Unfortunately, in the current study, this information was recorded in a minority of patients for whom 16G to 18G needles were used, with no differences in terms of diagnostic concordance. Nevertheless, we believe this observation needs to be confirmed in a larger prospective cohort.
To conclude, CNB has become a standard practice over the years, and multiple studies have shown its effectiveness in diagnosing cancers, including lymphomas. Our study extends previous reports and shows that CNB appears easy to perform and is minimally invasive, as well as suitable for lymphoma diagnosis in most instances11-39,42; however, we found that this sampling procedure increases the risk of nondefinitive diagnoses. Moreover, misdiagnoses of benign lesions with CNB are not negligible and have to be considered because they might dramatically affect patients’ care. These significant limitations of CNB may be balanced by expert review. Otherwise, pathologists who feel uncertain about a diagnosis of lymphoma with CNB should recommend a lymph node surgical excision.
Acknowledgments
The authors thank the Lymphopath consortium: J. Brière, C. Copie, E. Poullot, and N. Ortonne (Hôpital Henri Mondor, AP-HP), M. Battistella and V. Meignin (Hôpital Saint-Louis, AP-HP), J. Bruneau (Hôpital Necker, AP-HP), D. Damotte and B. Burroni (Hôpital Cochin, AP-HP), B. Fabiani and J.F. Fléjou (Hôpital Saint Antoine, AP-HP), F. Charlotte and E. Labouyrie (Hôpital La Pitié Salpêtrière, AP-HP), A. Martin and A. Levy (Hôpital Avicenne, AP-HP), J. Bosq and P. Dartigues (Institut Gustave Roussy), L. Lamant (CHU de Toulouse IUCT Oncopole), V. Costes Martineau (CHU de Montpellier), B. Vergier and P. Melard (CHU de Bordeaux), I. Soubeyran (Institut Bergonié), J. Fontaine, S. Isaac, B. Balme, and O. Harou (CHU de Lyon Sud), C. Chassagne-Clément, A. Decouvelaere, and A. Fouchardière (Centre Léon Bérard, Lyon), M. Peoc’h (CHU de Saint Etienne), A. Ledoux-Pilon (CHU de Clermont-Ferrand), L. Mescam and S. Taix (Institut Paoli Calmettes, Marseille), J.F. Michiels, I. Peyrotte, O. Vire, B. Chetaille, and M. Benchetrit (CHU de Nice), C. Bossard (CHU de Nantes), M.C. Rousselet and A. Croué (CHU d’Angers), P. Tas, F. Llamas, and C. Le Naourès (CHU de Rennes), F. Arbion and A. de Muret (CHU de Tours), I. Quintin-Roué (CHU de Brest), S. Humez and M. Crinquette (CHU de Lille), H. Sevestre, C. Cordonnier, and D. Chatelain (CHU d’Amiens), L. Veresezan and F. Drieux (Centre Henri Becquerel), A. Nicolae (CHU de Strasbourg), I. Bededjian (CHU de Besançon-Dijon), H. Busby-Verner (CHU de Nancy), A. Marchal, M. Bayaram, and H. Schwartz (CHU de Reims), and M. Delage, B. Petit, and A. Guyot (CHU de Limoges). They also thank N. Curdy for drawing the visual abstract. The authors also heartily thank all the French referral pathologists for their active contribution to the Lymphopath network.
Authorship
Contribution: C.S., P.G., P.B., and C.L. conceived the study; C.L., P.B., P.G., E.P., N.A., V.F., M.P., A.T.-G., T.-J.M., L.X., L.M., R.D., V.L.-S., M.C.-C., A.M., and M.-P.C. provided the patient data for the study; C.S., C.C., A.L., B.C., and C.L. gathered the data and conducted the analyses; and C.S., C.C., P.G., P.B., and C.L. contributed to data interpretation and writing of the manuscript; and all authors reviewed the final manuscript, and all authors had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Camille Laurent, Département de Pathologie, Institut Universitaire du Cancer de Toulouse-Oncopole, 1 ave Irène Joliot-Curie 31059 Toulouse France; Université Paul-Sabatier, CRCT Inserm U1037, Toulouse, F-31400 France; e-mail: laurent.c@chu-toulouse.fr; and Philippe Gaulard, Département de Pathologie, Assistance Publique – Hôpitaux de Paris (AP-HP), Groupe Hospitalier Henri Mondor–Albert Chenevier, Créteil, France; INSERM U955, Université Paris-Est, Créteil, France; e-mail: philippe.gaulard@aphp.fr.
References
Author notes
Individual participant data are not available to share. Participating study groups should be contacted directly for the original data.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal