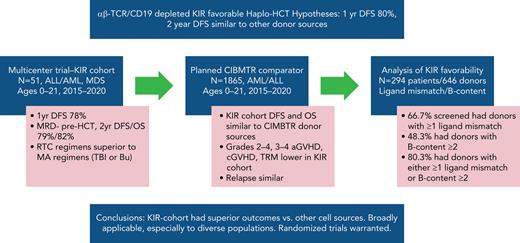
Key Points
Two-year DFS/OS of children with leukemia/MDS (MRD <0.01%) were 79%/82%, with rates of transplant-related mortality and chronic GVHD <10%.
Non-radiation containing reduced-toxicity approaches led to superior DFS/OS compared with traditional myeloablative approaches.
Abstract
We performed a prospective multicenter study of T-cell receptor αβ (TCR-αβ)/CD19–depleted haploidentical hematopoietic cell transplantation (HCT) in children with acute leukemia and myelodysplastic syndrome (MDS), to determine 1-year disease-free survival (DFS) and compare 2-year outcomes with recipients of other donor cell sources. Fifty-one patients aged 0.7 to 21 years were enrolled; donors were killer immunoglobulin-like receptor (KIR) favorable based on ligand mismatch and/or high B content. The 1-year DFS was 78%. Superior 2-year DFS and overall survival (OS) were noted in patients <10 years of age, those treated with reduced toxicity conditioning (RTC) rather than myeloablative conditioning, and children with minimal residual disease <0.01% before HCT. Multivariate analysis comparing the KIR-favorable haploidentical cohort with controls showed similar DFS and OS compared with other donor cell sources. Multivariate analysis also showed a marked decrease in the risk of grades 2 to 4 and 3 to 4 acute graft versus host disease (aGVHD), chronic GVHD, and transplant-related mortality vs other donor cell sources. Ethnic and racial minorities accounted for 53% of enrolled patients, and data from a large cohort of recipients/donors screened for KIR showed that >80% of recipients had a KIR-favorable donor by our definition, demonstrating that this approach is broadly applicable to groups often unable to find donors. This prospective, multicenter study showed improved outcomes using TCR-αβ/CD19–depleted haploidentical donors using RTC for children with acute leukemia and MDS. Randomized trials comparing this approach with matched unrelated donors are warranted. This trial was registered at https://clinicaltrials.gov as #NCT02646839.
Introduction
The importance of strategic mismatch of killer immunoglobulin-like receptors (KIRs) between donor and recipients of allogeneic hematopoietic cell transplantation (HCT) has been debated extensively after early investigations in haploidentical HCT of patients with acute myeloid leukemia (AML) showed that specific combinations of KIR molecules in donors and recipients decreased relapse and lowered transplant-related mortality (TRM).1-3 Although the effect has been absent or equivocal in some studies,4-6 several studies have shown benefits in both pediatric and adult recipients of cells from a variety of donor sources.7-10 Methods of choosing optimal donors based on KIR differences have focused on 2 broad approaches: (1) the absence of KIR inhibitory ligands in a recipient with the presence of the KIR inhibitory molecule in the donor, allowing for the natural killer (NK) activity against recipient cancer cells to proceed, and (2) the presence of higher levels of KIR molecules that license or increase the likelihood of NK-cell activation against recipient cells.
The first category of KIR effect has been termed “missing ligand” and is routinely assessed by testing for the presence or absence of the C1, C2, and Bw4 inhibitory ligands on patients, while assessing donors for the presence of their inhibitory molecule binding partners 2DL2 and 2DL3, 2DL1, and 3DL1, respectively.11,12 There are many ways of assessing licensing of NK activity by KIR molecules. One example is a model put forward by Cooley who used a combination of types of KIR molecules (A vs B), as well as location of the KIR molecules (centromeric vs telomeric).9 They noted that in recipients of HCT for AML, those receiving donors with higher B content had the most protection against relapse. Donor/recipient missing ligand and donor B content according to Cooley score can be easily calculated (https://www.ebi.ac.uk/ipd/kir/donor_b_content.html) based on donor KIR typing and then used to choose donors for prospective trials.
The bulk of the HCT literature on KIR has focused on AML in adults, and KIR effects in adult patients with acute lymphocytic leukemia (ALL) have usually been limited or absent.9,13 By contrast, multiple investigators have shown a beneficial effect of KIR mismatching in pediatric patients with ALL who undergo HCT,14,15 with a prominent role played by NK cells in the control of both diseases.16,17 Because a large portion of HCT for hematological malignancies in pediatrics occurs in patients with ALL, demonstration of a beneficial KIR effect in pediatric ALL would be important to the pediatric HCT field.18 Single-center work by Leung and colleagues at St Jude Children’s Hospital showed that when using KIR-favorable donors, survival outcomes with ex vivo T-cell–depleted haplotransplantation approached or exceeded those obtained using other donor types (matched sibling and unrelated).19 These outstanding outcomes in a pediatric population included all hematological malignancies, but a sizable percentage were transplanted for ALL.
With these preliminary data in hand, we hypothesized that in a multicenter setting, 1-year disease-free survival (DFS) after T-cell receptor-αβ (TCR-αβ)/CD19–depleted haploidentical HCT for children and young adults up to age 21 with complete response 1 (CR1)/CR2 ALL/AML/myelodysplastic syndrome (MDS) would be 80% and would compare favorably with other donor types if concomitant controls were used. This article describes primary outcomes for the ONC1401 trial run through the Pediatric Transplantation and Cellular Therapy Consortium (PTCTC).
Methods
PTCTC ONC1401 was a phase 2 trial conducted at 12 institutions of the PTCTC from January 2016 through July 2021. The study was approved by all local institutional review boards. Written informed consent was obtained from all parents and/or legal guardians in accordance with the Declaration of Helsinki.
Patient eligibility
Patients with ALL, AML, or MDS who were in CR1 or CR2 were eligible if they were <22 years of age with adequate organ function and had an eligible KIR-favorable haploidentical donor. The KIR-favorability assessment was performed in a single Clinical Laboratory Improvement Amendments–approved laboratory at Children’s Hospital of Philadelphia. KIR favorability was defined as B content according to the Cooley Scale of ≥29 or inhibitory ligand mismatch by DNA typing or both.
Additional disease-specific criteria were as follows: for ALL, high-risk in CR1 eligible for HCT. Example CR1 indications: induction failure (>5% blasts by morphology on postinduction bone marrow [BM]), minimal residual disease (MRD) by flow cytometry >0.01% after consolidation, hypodiploidy (<44 chromosomes), persistent or recurrent cytogenetic or molecular evidence of disease during therapy requiring additional therapy after induction to achieve remission (eg, persistent molecular BCR-ABL positivity). ALL in CR2 indications were B cell, early (≤36 months from initiation of therapy) BM relapse; late BM relapse with MRD >0.1% by flow cytometry after first induction; T-cell or Ph+ with BM relapse at any time; very early (<18 months from initiation of therapy) isolated extramedullary relapse (T cell or B cell). Indications for AML were high-risk AML defined as monosomy 5, del 5q, monosomy 7, M6, M7, t(6;9), FLT3-ITD, or patients who have ≥25% blasts by morphology after induction, or failure to achieve CR after 2 courses of therapy. Also, patients with ≥0.1% MRD or evidence of progressive extramedullary disease after induction chemotherapy. These patients achieved morphologic remission before transplant. Any patients with AML in CR2 were allowed. Indications for MDS: any 2001 World Health Organization classification subtype was eligible (cytogenetic/molecular lesions of patients; supplemental Table 1, available on the Blood Web site); eligibility criteria (full study protocol) are included as supplemental Data.
Study procedures
Patients received a preparative regimen with either traditional myeloablative (MAC) or reduced toxicity (RTC) conditioning, less-intensive myeloablative approaches using agents associated with lower rates of toxicity. The 2 MAC regimen options included rabbit antithymocyte globulin (rATG; thymoglobulin) 3 mg/kg from day −12 to day −10 (total, 9 mg/kg), total body irradiation (TBI) 200 cGy twice daily from day −8 to day −6 (total, 1200 cGy; TBI-MAC), or busulfan from day −9 to day −6 (targeting an area under the curve of 60 to 95 mg/L per hour; Bu-MAC), with both regimens also including thiotepa (thio) 5 mg/kg on days −5 and −4 (total 10 mg/kg) and cyclophosphamide (cy) 60 mg/kg on days −3 and −2 (total 120 mg/kg). RTC regimen choices included (1) rATG 3 mg/kg from days −12 to −10 (total, 9 mg/kg), fludarabine (flu) 40 mg/m2 from days −9 to day −5 (total, 200 mg/m2), thio 5 mg/kg twice daily on day −4 (total, 10 mg/m2), melphalan (mel) 70 mg/m2 on days −3 and −2 (total, 140 mg/m2), or (2) total lymphoid irradiation 200 cGy twice daily on day −9, 200 cGy daily from day −8 to day −7 (total 800 cGy), flu 30 mg/m2 from day −8 to day −4 (total 150 mg/m2), cy 60 mg/kg on day −6, thio 5 mg/kg twice daily on day −4 (total, 10 mg/kg), and mel 70 mg/m2 on days −3 and −2 (total 140 mg/m2). The choice of regimen was based on center, investigator, and patient preference.
All haploidentical grafts were peripheral blood stem cells (PBSCs) processed fresh using Miltenyi CliniMACS TCR-αβ/CD19 depletion using infused graft cell processing targets as follows: CD34+ goal ≥10 × 106/kg, TCR-αβ+CD3+ goal <1 × 105/kg, CD19+ goal <1 × 105/kg, with rituximab 375 mg/m2 given on day +1 if CD19+ cells exceeded 1 × 105/kg. CD34+ selection and a second infusion within a day was allowed to optimize the CD34+ cell dose. No pharmacological graft-versus-host disease (GVHD) prophylaxis was used. Granulocyte-colony stimulating factor (G-CSF) was used rarely, according to local practice, but only after days +14 to +21.
Statistical analysis
The primary objective of this phase 2 trial was to prospectively assess DFS (events: relapse and death) of KIR-favorable haploidentical HCT using ex vivo TCR αβ+CD3+/CD19+–depleted grafts in children with high-risk ALL, AML, and MDS. Secondary objectives included assessing rates of relapse, TRM, GVHD, event-free survival (EFS; events: relapse, death, and rejection), and overall survival (OS) and comparing these outcomes with concurrently enrolled children receiving myeloablative HCT from matched sibling, matched unrelated, 7 of 8 HLA-mismatched unrelated, and cord blood cell sources. Of 1865 recipients in the CIMBTR (Center for International Blood and Marrow Transplant Research) cohort, MRD data were available on 1745 patients. Additional secondary objectives included assessment of outcomes by MRD status and identification of risk factors for poor outcome, including a comparison of preparative approaches.
Cumulative incidences of GVHD, relapse, and TRM were considered to accommodate for competing risks. Cox proportional hazard analysis for EFS, DFS, and OS and the proportional cause-specific hazards model for GVHD, relapse, and TRM were used to identify prognostic factors via forward stepwise selection. The proportional hazard assumption for each variable was examined by testing whether its coefficient was constant over time. Covariates with a P < .05 were considered significant. Interactions among significant covariates were also examined. The variables for the regression analysis include race, ethnicity, donor/recipient sex match, donor/recipient cytomegalovirus serostatus, disease type, disease status, MRD status, and performance status. Adjusted survival and cumulative incidences were calculated based on the final regression model for each outcome.9,10 Kaplan-Meier estimates and cumulative incidence were calculated for the analysis restricted to the clinical trial data. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and R version 4.0 (Vienna, Austria).
Results
Demographics of the KIR-favorable haploidentical and CIBMTR comparator cohorts
Table 1 shows details of the prospectively enrolled phase 2 KIR-favorable haploidentical cohort (KIR cohort) and the CIBMTR cohort. The median age of patients on the trial was 11.7 (range, 0.7-21.8) years, whereas the median donor age was 35 (6-61) years. Although most of the haploidentical donors were parents (maternal, 43%; paternal, 57%), 12 (24%) were siblings and 2 were other related donors. The majority (59%) of the patients underwent HCT for ALL with a fairly even mix of CR1 and CR2. Only 5 patients had MDS, all of whom had advanced MDS. Because of the low number of patients with MDS in the phase 2 cohort, we did not include them in the comparative analysis with the CIBMTR comparator cohort. The CIBMTR cohort included all transplants at US centers in patients <22 years of age (median 12.2; range, 0.34-21.96) with ALL and AML, with HLA-identical sibling donors, well-matched (8 of 8 alleles) or partially matched (7 of 8 alleles) unrelated donors (URDs), or unrelated cord blood (UCB) during the period of the trial (2015 through 2020), and was limited to myeloablative procedures by CIBMTR consensus definition20 for first HCT in CR1 or CR2.
Patient demographics for both the phase 2 cohort and the CIBMTR comparator cohort
| Characteristic . | Phase 2 cohort (n = 51) . | CIBMTR cohort (n = 1865) . |
|---|---|---|
| Patients | ||
| Median age at HCT, y (range) | 11.73 (0.67-21.79) | 12.24 (0.34-21.96) |
| Sex, n (%) | ||
| Male | 31 (61) | 1098 (60) |
| Female | 20 (39) | 730 (40) |
| Donor, n (%) | ||
| Median age, y (range) | 35 (6-61) | 23.4 (0-60) |
| Donor sex | ||
| Male | 29 (57) | 1043 (57) |
| Female | 22 (43) | 769 (42) |
| Data missing | — | 16 (1) |
| Donor/patient relations, n (%) | ||
| Sex mismatch (F≥M) | 12 (23.5) | 446 (24.4) |
| Donor relationship, n (%) | ||
| Parent; maternal | 37 (72.5); 16 (43) | 0 |
| Sibling | 12 (23.5) | 527 (29) |
| Other related | 2 (4) | 0 |
| Unrelated | 0 | 886 (48) |
| CB | 0 | 415 (23) |
| Disease status, n (%) | ||
| ALL CR1 | 16 (31.4) | 475 (26) |
| ALL CR2 | 14 (27.5) | 487 (26.6) |
| AML CR1 | 11 (21.6) | 617 (33.8) |
| AML CR2 | 5 (9.8) | 249 (13.6) |
| MDS | 5 (9.8) | 0 |
| Cell source | ||
| Haplo (5/10, 6/10, 7/10, 8/10) | 51 | 0 |
| Matched sibling donor | 0 | 527 (29) |
| MURD (9/10, 10/10) | 0 | 670 (37) |
| MMURD (7/8) | 0 | 216 (12) |
| CB 8/8 | 0 | 47 (3) |
| CB 7/8 | — | 75 (4) |
| CB ≤6/8 | — | 199 (11) |
| CB multidonor | — | 82 (4) |
| CB missing | — | 5 (<1) |
| CMV | ||
| Patient | ||
| Positive | 27 (53) | 1056 (58) |
| Negative | 24 (47) | 564 (31) |
| Data missing | 0 | 208 (11) |
| Donor | ||
| Positive | 27 (53) | 734 (40) |
| Negative | 24 (47) | 886 (48) |
| Data missing | 0 | 208 (11) |
| Conditioning regimens, n (%) | ||
| MAC | 27 (53) | 1828 |
| TBI | 16 (31) | — |
| Busulfan | 11 (22) | — |
| RTC | 24 (47) | 0 |
| rATG/flu/mel/thio | 22 (43) | — |
| TLI | 2 (4) | — |
| NK alloreactivity | ||
| Ligand mismatch (yes/no) | 44/7 | — |
| B-content value 0-1 vs ≥2 | 20/31 | — |
| Race | ||
| Black | 5 (10) | 141 (7.7) |
| Asian | 9 (17.6) | 151 (8.3) |
| White | 24 (47) | 1344 (73.5) |
| Other | 13 (25.5) | 192 (10.5) |
| Ethnicity | ||
| Non-Hispanic | 24 (47) | 1196 (65.4) |
| Hispanic | 23 (45.1) | 554 (30.3) |
| Other | 4 (7.8) | 78 (4.3) |
| Follow-up | ||
| Median follow-up, d (range) | 609 (29-1772) | 740 (42-1961) |
| Median cell dose infused/recipient weight (range) ×106/kg | ||
| CD34+ cells (n = 51) | 12.19 (2.57-36.4) | — |
| TCR-αβ cells (n = 51) | 0.02845 (0-0.418) | — |
| TCR-γδ cells (n = 51) | 7.739 (0.1077-1810) | — |
| NK cells (n = 47) | 41.13 (3.41-193.3) | — |
| CD20+ cells (n = 51) | 0.06435 (0.007-2.756) | — |
| Characteristic . | Phase 2 cohort (n = 51) . | CIBMTR cohort (n = 1865) . |
|---|---|---|
| Patients | ||
| Median age at HCT, y (range) | 11.73 (0.67-21.79) | 12.24 (0.34-21.96) |
| Sex, n (%) | ||
| Male | 31 (61) | 1098 (60) |
| Female | 20 (39) | 730 (40) |
| Donor, n (%) | ||
| Median age, y (range) | 35 (6-61) | 23.4 (0-60) |
| Donor sex | ||
| Male | 29 (57) | 1043 (57) |
| Female | 22 (43) | 769 (42) |
| Data missing | — | 16 (1) |
| Donor/patient relations, n (%) | ||
| Sex mismatch (F≥M) | 12 (23.5) | 446 (24.4) |
| Donor relationship, n (%) | ||
| Parent; maternal | 37 (72.5); 16 (43) | 0 |
| Sibling | 12 (23.5) | 527 (29) |
| Other related | 2 (4) | 0 |
| Unrelated | 0 | 886 (48) |
| CB | 0 | 415 (23) |
| Disease status, n (%) | ||
| ALL CR1 | 16 (31.4) | 475 (26) |
| ALL CR2 | 14 (27.5) | 487 (26.6) |
| AML CR1 | 11 (21.6) | 617 (33.8) |
| AML CR2 | 5 (9.8) | 249 (13.6) |
| MDS | 5 (9.8) | 0 |
| Cell source | ||
| Haplo (5/10, 6/10, 7/10, 8/10) | 51 | 0 |
| Matched sibling donor | 0 | 527 (29) |
| MURD (9/10, 10/10) | 0 | 670 (37) |
| MMURD (7/8) | 0 | 216 (12) |
| CB 8/8 | 0 | 47 (3) |
| CB 7/8 | — | 75 (4) |
| CB ≤6/8 | — | 199 (11) |
| CB multidonor | — | 82 (4) |
| CB missing | — | 5 (<1) |
| CMV | ||
| Patient | ||
| Positive | 27 (53) | 1056 (58) |
| Negative | 24 (47) | 564 (31) |
| Data missing | 0 | 208 (11) |
| Donor | ||
| Positive | 27 (53) | 734 (40) |
| Negative | 24 (47) | 886 (48) |
| Data missing | 0 | 208 (11) |
| Conditioning regimens, n (%) | ||
| MAC | 27 (53) | 1828 |
| TBI | 16 (31) | — |
| Busulfan | 11 (22) | — |
| RTC | 24 (47) | 0 |
| rATG/flu/mel/thio | 22 (43) | — |
| TLI | 2 (4) | — |
| NK alloreactivity | ||
| Ligand mismatch (yes/no) | 44/7 | — |
| B-content value 0-1 vs ≥2 | 20/31 | — |
| Race | ||
| Black | 5 (10) | 141 (7.7) |
| Asian | 9 (17.6) | 151 (8.3) |
| White | 24 (47) | 1344 (73.5) |
| Other | 13 (25.5) | 192 (10.5) |
| Ethnicity | ||
| Non-Hispanic | 24 (47) | 1196 (65.4) |
| Hispanic | 23 (45.1) | 554 (30.3) |
| Other | 4 (7.8) | 78 (4.3) |
| Follow-up | ||
| Median follow-up, d (range) | 609 (29-1772) | 740 (42-1961) |
| Median cell dose infused/recipient weight (range) ×106/kg | ||
| CD34+ cells (n = 51) | 12.19 (2.57-36.4) | — |
| TCR-αβ cells (n = 51) | 0.02845 (0-0.418) | — |
| TCR-γδ cells (n = 51) | 7.739 (0.1077-1810) | — |
| NK cells (n = 47) | 41.13 (3.41-193.3) | — |
| CD20+ cells (n = 51) | 0.06435 (0.007-2.756) | — |
CMV cytomegalovirus; TLI, total lymphoid irradiation.
The phase 2 cohort had substantially higher numbers of ethnic minorities compared with the CIBMTR cohort: non-White 53% vs 26.5% (P < .0001), Hispanic 45% vs 30% (P = .02; Table 1).
Engraftment and immune reconstitution/effect of rituximab
CD34+ cell doses were high in the KIR cohort, with a median 12 × 106 CD34+ cells/kg recipient weight. Neutrophil engraftment was rapid (median day +14; range, 8-19). The overall rate of rejection was low (n = 4; 7.8%). Rejection occurred mostly in myeloid patients (2 AML, 1 MDS, and 1 ALL) and all received the rATG/flu/thio/mel approach. Three of the patients were at high risk for rejection, 2 due to a prolonged period without chemotherapy before HCT (2 to 4 months), and 1 due to MDS with high-risk cytogenetics, no pre-HCT chemotherapy, and low cell dose (4.83 × 106/kg CD34+/kg, below our target of 10 × 106/kg). All were successfully engrafted after rescue HCT using a different donor (1 mismatched URD, 1 cord, and 2 haplo).
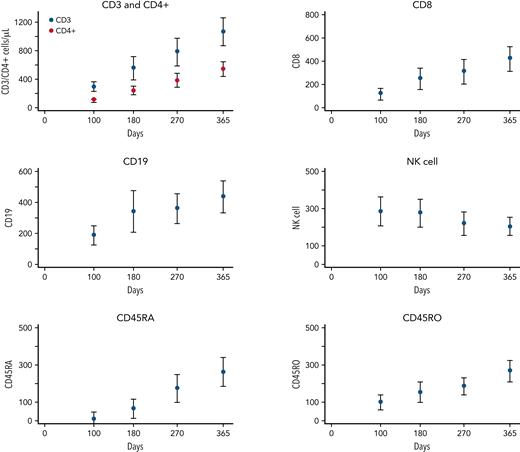
Immune reconstitution was notable for normalization of NK cell counts by day 100, with a mean of 282/μL (95% confidence interval [CI], 204-360) followed by stable NK counts through the first year. T- and B-cell counts were a mean CD3 and CD19 of 284/μL (95% CI, 284-354) and 184/μL (95% CI, 120-248) at day 100 and 554/μL (95% CI, 390-717) and 339/μL (95% CI, 202-476) at 6 months, with continued increases over the first year after HCT. CD45RA and CD45RO counts were a mean of 1/μL (95% CI, 0-44) and 95/μL (95% CI, 55-136) at day 100; 61/μL (95% CI, 17-103) and 150/μL (95% CI, 109-191) at 6 months; and 171/μL (95% CI, 128-214) and 183/μL (95% CI, 142-224) at 9 months after HCT (Figure 1).
Lymphocyte recovery over the first year after transplant for CD3, CD4, CD8, CD19, NK cells, CD45RA, and CD45RO. The y-axes are expressed in cells per microliter, and the x-axes are days after HCT infusion.
Lymphocyte recovery over the first year after transplant for CD3, CD4, CD8, CD19, NK cells, CD45RA, and CD45RO. The y-axes are expressed in cells per microliter, and the x-axes are days after HCT infusion.
An analysis of patients who received rituximab after infusion for CD20+ counts above 1 × 105/kg in the depleted product (24 received/27 did not) showed no differences in time to cellular immune recovery or cessation of IVIg. GVHD and survival outcomes were similarly not affected by rituximab infusion. One patient who did not receive rituximab prophylactically developed CNS posttransplant lymphoproliferative disorder that required rituximab, local radiation therapy, and Epstein-Barre virus–specific T-cells to control.
Survival in the KIR cohort
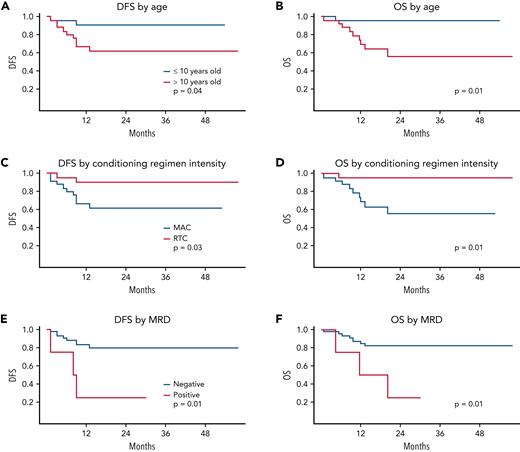
One-year DFS in the KIR cohort was 78% (95% CI, 66% to 89%), with a 2-year EFS, DFS, and OS of 69% (95% CI, 56% to 82%), 75% (95% CI, 63% to 88%), and 75% (95% CI, 63% to 88%), respectively. Univariate risk analysis showed that several factors were predictive of survival. Age ≤10 years, RTC vs MAC, and flow MRD <0.01% resulted in significant improvements in survival (P = .03, .03, and .02, respectively; Figure 2). The protocol included 2 traditional MAC approaches and 2 RTC approaches to accommodate patients not eligible for traditional MAC; however, because the RTC approaches are myeloablative and had been the preferred approaches in the St Jude single-center data,19 many centers chose RTC over traditional MAC for patients eligible for both. The higher rate of failure in the traditional MAC regimens was a mixture of both relapse and TRM: 5 of 27 (18.5%; 95% CI, 6% to 38%) relapsed and 5 of 27 (18.5%; 95% CI, 6% to 38%) experienced TRM with MAC vs 3 of 24 (12.5%; 95% CI, 3% to 32%) experiencing relapse and 0% (95% CI, 0% to 14%) TRM with RTC approaches.
DFS and OS. Survival statistics by age (A-B), conditioning regimen (C-D), and MRD status (E-F).
DFS and OS. Survival statistics by age (A-B), conditioning regimen (C-D), and MRD status (E-F).
Although half of the flow MRD-positive patients were treated with the MAC and half with the RTC regimens, MRD-positive patients were rare (n = 6; 12%) and, as anticipated, did poorly with both approaches (4 of 6 relapsed, 3 of 6 died; Figure 2E-F). To remove possible confounding of the imbalance of high-risk MRD-positive patients to the of MAC vs RTC outcomes comparison, we performed a subanalysis of outcomes in patients who were flow MRD <0.01% before HCT. Notably, 2-year DFS and OS for patients were 79% (95% CI, 66% to 92%) and 82% (95% CI, 69% to 94%), respectively (Figure 3C-D). Superior survival outcomes for RTC vs MAC held in the MRD-negative cohort, with 2-year DFS of 90% (95% CI, 77% to 100%) vs 60% (95% CI, 40% to 81%), and OS of 95% (95% CI, 86% to 100%) and 55% (95% CI, 32% to 79%) with RTC vs MAC, respectively (P = .03 for both). ALL patients were our largest population; 16 patients with ALL were treated with a TBI regimen and 14 non-TBI, all flow MRD <0.01% at HCT. Treatment failed in 3 of the 16 patients conditioned with TBI (19%: 2 relapsed, 1 TRM) and in 2 of the 14 not treated with TBI (14%: 1 relapse, 1 TRM).
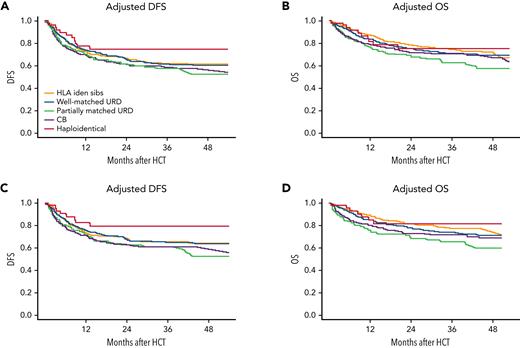
Adjusted DFS and OS. Adjusted statistics of the full phase 2 cohort vs CIBMTR cohorts (A-B) and the flow MRD negative before HCT phase 2 vs CIBMTR cohorts (C-D).
Adjusted DFS and OS. Adjusted statistics of the full phase 2 cohort vs CIBMTR cohorts (A-B) and the flow MRD negative before HCT phase 2 vs CIBMTR cohorts (C-D).
Analysis of survival outcomes in the KIR and CIBMTR cohorts
Multivariable analyses for survival end points comparing the KIR cohort with cell sources from the CIBMTR cohort is presented in Table 2. A subanalysis of patients in both cohorts coming to HCT with flow MRD <0.01% is also included in Table 2. There was a trend toward improved DFS for KIR cohort recipients compared with recipients of partially matched unrelated donors (URDs) and UCB, which strengthened in the MRD-negative analysis (partially matched URD hazard ratio [HR], 2.06; 95% CI, 0.98-4.3; P = .057) and UCB HR 2.0 (95% CI, 0.97-4.1; P = .06). OS was improved the first 4 months after HCT compared with other donor cell sources in both the overall and MRD-negative cohort analyses (P = .0001 and .0002, respectively), but was similar after 4 months. Figure 3 shows the DFS and OS comparisons between the KIR cohort and recipients of other donor cell sources for both the full cohorts and the pre-HCT MRD-negative cohorts.
Combined analyses of the full and MRD-negative KIR cohorts with the full and MRD-negative CIBMTR cohorts for DFS/OS
| Outcome . | HR . | 95% CI, lower limit . | 95% CI, upper limit . | P . | Overall P . | n . |
|---|---|---|---|---|---|---|
| KIR cohort and CIBMTR cohort | ||||||
| DFS | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .1312 | 46 |
| HLA-identical sibling | 1.434 | 0.778 | 2.64 | .2478 | 526 | |
| Well-matched URD | 1.448 | 0.788 | 2.661 | .2337 | 665 | |
| Partially matched URD | 1.745 | 0.929 | 3.277 | .0834 | 216 | |
| CB | 1.728 | 0.937 | 3.189 | .0799 | 412 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .0305 | 569 |
| Non-Hispanic | 0.798 | 0.672 | 0.948 | .0101 | 1215 | |
| Data missing | 0.758 | 0.502 | 1.145 | .1879 | 81 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0009 | 876 |
| ALL | 0.76 | 0.647 | 0.894 | .0009 | 989 | |
| Disease status (≤8 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0004 | 1114 |
| CR2 | 1.426 | 1.171 | 1.737 | .0004 | 751 | |
| Disease status (>8 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0852 | 774 |
| CR2 | 0.781 | 0.59 | 1.035 | .0852 | 499 | |
| OS | ||||||
| Donor type (≤4 mo) | ||||||
| Haploidentical | 1.00 | — | — | — | .0001 | 46 |
| HLA-identical sibling | 2.024 | 0.274 | 14.975 | .4897 | 527 | |
| Well-matched URD | 2.67 | — | 19.485 | .3327 | 670 | |
| Partially matched URD | 4.783 | — | 35.521 | .126 | 216 | |
| CB | 5.567 | 0.769 | 40.279 | .089 | 415 | |
| Donor type (>4 mo) | ||||||
| Haploidentical | 1.00 | 0.408 | 1.619 | .556 | .2449 | 45 |
| HLA-identical sibling | 0.813 | 0.463 | 1.813 | .8015 | 491 | |
| Well-matched URD | 0.916 | 0.585 | 2.439 | .625 | 619 | |
| Partially matched URD | 1.195 | 0.409 | 1.652 | .5813 | 191 | |
| CB | 0.822 | 0.409 | 1.652 | .5813 | 356 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .01 | 574 |
| Non-Hispanic | 0.756 | 0.618 | 0.925 | .0066 | 1218 | |
| Data missing | 0.58 | 0.34 | 0.989 | .0454 | 82 | |
| Disease (≤6 mo) | ||||||
| AML | 1.00 | .8471 | 882 | |||
| ALL | 1.03 | 0.765 | 1.385 | .8471 | 992 | |
| Disease (>6 mo) | ||||||
| AML | 1.00 | — | — | — | <.0001 | 741 |
| ALL | 0.53 | 0.411 | 0.682 | <.0001 | 824 | |
| Disease status (≤5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0003 | 1119 |
| CR2 | 1.813 | 1.317 | 2.496 | .0003 | 755 | |
| Disease status (>5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .5978 | 1018 |
| CR2 | 0.936 | 0.734 | 1.195 | .5978 | 652 | |
| MRD−KIR cohort and MRD−CIBMTR cohort | ||||||
| DFS | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .1327 | 42 |
| HLA-identical sibling | 1.636 | 0.797 | 3.355 | .1795 | 378 | |
| Well-matched URD | 1.617 | 0.792 | 3.303 | .1869 | 478 | |
| Partially matched URD | 2.057 | 0.978 | 4.327 | .0574 | 149 | |
| CB | 1.995 | 0.971 | 4.098 | .0601 | 298 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0126 | 609 |
| ALL | 0.782 | 0.645 | 0.949 | .0126 | 736 | |
| OS | ||||||
| Donor type (≤4 mo) | ||||||
| Haploidentical | 1.00 | — | — | — | .0002 | 42 |
| HLA-identical sibling | 1.959 | 0.261 | 14.731 | .5134 | 379 | |
| Well-matched URD | 2.219 | 0.299 | 16.486 | .4361 | 480 | |
| Partially matched URD | 6.092 | 0.815 | 45.541 | .0783 | 149 | |
| CB | 5.046 | 0.69 | 36.886 | .1108 | 300 | |
| Donor type (>4 mo) | ||||||
| Haploidentical | 1.00 | — | — | — | .4106 | 41 |
| HLA-identical sibling | 0.87 | 0.371 | 2.043 | .7497 | 354 | |
| Well-matched URD | 1.15 | 0.497 | 2.658 | .7441 | 443 | |
| Partially matched URD | 1.374 | 0.564 | 3.344 | .4841 | 126 | |
| CB | 1.061 | 0.45 | 2.499 | .8922 | 258 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .0314 | 406 |
| Non-Hispanic | 0.728 | 0.567 | 0.935 | .0129 | 886 | |
| Data missing | 0.613 | 0.319 | 1.181 | .1436 | 58 | |
| Disease (≤6 mo) | ||||||
| AML | 1.00 | — | — | — | .4472 | 612 |
| ALL | 1.152 | 0.799 | 1.661 | .4472 | 738 | |
| Disease (>6 mo) | ||||||
| AML | 1.00 | — | — | — | <.0001 | 511 |
| ALL | 0.49 | 0.354 | 0.677 | <.0001 | 611 | |
| Disease status (≤5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0081 | 784 |
| CR2 | 1.686 | 1.145 | 2.482 | .0081 | 566 | |
| Disease status (>5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0512 | 711 |
| CR2 | 0.73 | 0.533 | 1.002 | .0512 | 492 |
| Outcome . | HR . | 95% CI, lower limit . | 95% CI, upper limit . | P . | Overall P . | n . |
|---|---|---|---|---|---|---|
| KIR cohort and CIBMTR cohort | ||||||
| DFS | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .1312 | 46 |
| HLA-identical sibling | 1.434 | 0.778 | 2.64 | .2478 | 526 | |
| Well-matched URD | 1.448 | 0.788 | 2.661 | .2337 | 665 | |
| Partially matched URD | 1.745 | 0.929 | 3.277 | .0834 | 216 | |
| CB | 1.728 | 0.937 | 3.189 | .0799 | 412 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .0305 | 569 |
| Non-Hispanic | 0.798 | 0.672 | 0.948 | .0101 | 1215 | |
| Data missing | 0.758 | 0.502 | 1.145 | .1879 | 81 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0009 | 876 |
| ALL | 0.76 | 0.647 | 0.894 | .0009 | 989 | |
| Disease status (≤8 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0004 | 1114 |
| CR2 | 1.426 | 1.171 | 1.737 | .0004 | 751 | |
| Disease status (>8 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0852 | 774 |
| CR2 | 0.781 | 0.59 | 1.035 | .0852 | 499 | |
| OS | ||||||
| Donor type (≤4 mo) | ||||||
| Haploidentical | 1.00 | — | — | — | .0001 | 46 |
| HLA-identical sibling | 2.024 | 0.274 | 14.975 | .4897 | 527 | |
| Well-matched URD | 2.67 | — | 19.485 | .3327 | 670 | |
| Partially matched URD | 4.783 | — | 35.521 | .126 | 216 | |
| CB | 5.567 | 0.769 | 40.279 | .089 | 415 | |
| Donor type (>4 mo) | ||||||
| Haploidentical | 1.00 | 0.408 | 1.619 | .556 | .2449 | 45 |
| HLA-identical sibling | 0.813 | 0.463 | 1.813 | .8015 | 491 | |
| Well-matched URD | 0.916 | 0.585 | 2.439 | .625 | 619 | |
| Partially matched URD | 1.195 | 0.409 | 1.652 | .5813 | 191 | |
| CB | 0.822 | 0.409 | 1.652 | .5813 | 356 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .01 | 574 |
| Non-Hispanic | 0.756 | 0.618 | 0.925 | .0066 | 1218 | |
| Data missing | 0.58 | 0.34 | 0.989 | .0454 | 82 | |
| Disease (≤6 mo) | ||||||
| AML | 1.00 | .8471 | 882 | |||
| ALL | 1.03 | 0.765 | 1.385 | .8471 | 992 | |
| Disease (>6 mo) | ||||||
| AML | 1.00 | — | — | — | <.0001 | 741 |
| ALL | 0.53 | 0.411 | 0.682 | <.0001 | 824 | |
| Disease status (≤5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0003 | 1119 |
| CR2 | 1.813 | 1.317 | 2.496 | .0003 | 755 | |
| Disease status (>5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .5978 | 1018 |
| CR2 | 0.936 | 0.734 | 1.195 | .5978 | 652 | |
| MRD−KIR cohort and MRD−CIBMTR cohort | ||||||
| DFS | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .1327 | 42 |
| HLA-identical sibling | 1.636 | 0.797 | 3.355 | .1795 | 378 | |
| Well-matched URD | 1.617 | 0.792 | 3.303 | .1869 | 478 | |
| Partially matched URD | 2.057 | 0.978 | 4.327 | .0574 | 149 | |
| CB | 1.995 | 0.971 | 4.098 | .0601 | 298 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0126 | 609 |
| ALL | 0.782 | 0.645 | 0.949 | .0126 | 736 | |
| OS | ||||||
| Donor type (≤4 mo) | ||||||
| Haploidentical | 1.00 | — | — | — | .0002 | 42 |
| HLA-identical sibling | 1.959 | 0.261 | 14.731 | .5134 | 379 | |
| Well-matched URD | 2.219 | 0.299 | 16.486 | .4361 | 480 | |
| Partially matched URD | 6.092 | 0.815 | 45.541 | .0783 | 149 | |
| CB | 5.046 | 0.69 | 36.886 | .1108 | 300 | |
| Donor type (>4 mo) | ||||||
| Haploidentical | 1.00 | — | — | — | .4106 | 41 |
| HLA-identical sibling | 0.87 | 0.371 | 2.043 | .7497 | 354 | |
| Well-matched URD | 1.15 | 0.497 | 2.658 | .7441 | 443 | |
| Partially matched URD | 1.374 | 0.564 | 3.344 | .4841 | 126 | |
| CB | 1.061 | 0.45 | 2.499 | .8922 | 258 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .0314 | 406 |
| Non-Hispanic | 0.728 | 0.567 | 0.935 | .0129 | 886 | |
| Data missing | 0.613 | 0.319 | 1.181 | .1436 | 58 | |
| Disease (≤6 mo) | ||||||
| AML | 1.00 | — | — | — | .4472 | 612 |
| ALL | 1.152 | 0.799 | 1.661 | .4472 | 738 | |
| Disease (>6 mo) | ||||||
| AML | 1.00 | — | — | — | <.0001 | 511 |
| ALL | 0.49 | 0.354 | 0.677 | <.0001 | 611 | |
| Disease status (≤5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0081 | 784 |
| CR2 | 1.686 | 1.145 | 2.482 | .0081 | 566 | |
| Disease status (>5 mo) | ||||||
| CR1 | 1.00 | — | — | — | .0512 | 711 |
| CR2 | 0.73 | 0.533 | 1.002 | .0512 | 492 |
Bold P-values indicate statistically significant results.
Other factors notable for being independently associated with improved DFS include disease status (CR1; P = .0004), presence of ALL vs AML (P = .0009), and non-Hispanic ethnicity (P = .03).
Analysis of other significant outcomes: acute GVHD, chronic GVHD, relapse, and TRM
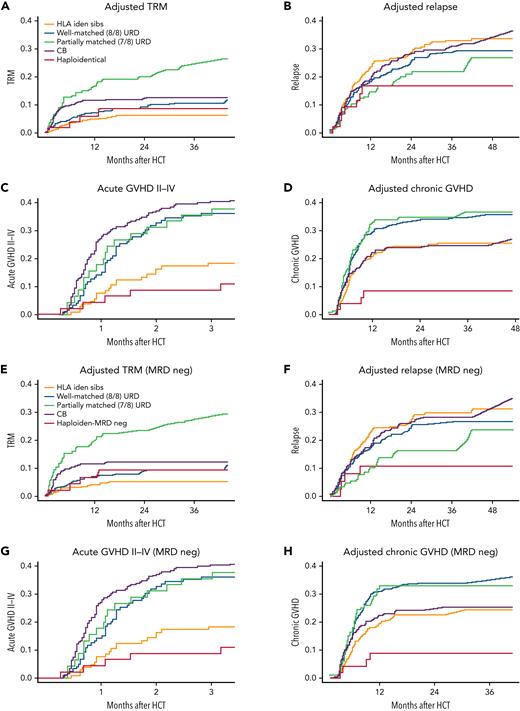
Table 3 shows multivariate analyses of the combined KIR and CIBMTR overall and MRD-negative cohorts for acute GVHD (aGVHD) grades 2 to 4, severe aGVHD grades 3 and 4, chronic GVHD (cGVHD), relapse, and TRM. Notably, there was a dramatic decrease in the occurrence of significant acute (grades 2-4), severe acute (grades 3-4), and cGVHD (overall P = .0001, = .0062, and < .0001, respectively) in recipients of TCR-αβ/CD19–depleted haploidentical HCT. Aside from comparisons of aGVHD with matched sibling donors, where the advantage is not definitive, HRs of 4- to 10-fold were noted in recipients of URD and UCB HCT. There was also a clear advantage for KIR cohort recipients in TRM compared with 7 of 8 mismatched URD recipients. Importantly, there was no difference noted in risk of relapse in the KIR cohort. Figure 4 shows the CI of grades 2 to 4 aGVHD, cGVHD, relapse, and TRM comparisons between the KIR cohort (red line) and recipients of other donor cell sources for both the full cohorts and the pre-HCT MRD-negative cohorts.
Comparative analyses of full and MRD-negative phase 2 with CIBMTR cohorts for GVHD, relapse, and TRM
| Outcome . | HR . | 95% CI, lower limit . | 95% CI, upper limit . | P . | Overall P . | n . |
|---|---|---|---|---|---|---|
| KIR cohort and CIBMTR cohort | ||||||
| aGVHD 2-4 | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0001 | 46 |
| HLA-identical sibling | 2.186 | 0.834 | 5.729 | .1117 | 104 | |
| Well-matched URD | 4.093 | 1.626 | 10.302 | .0028 | 119 | |
| Partially matched URD | 4.587 | 1.713 | 12.286 | .0024 | 45 | |
| CB | 4.907 | 2.00 | 12.039 | .0005 | 254 | |
| aGVHD 3-4 | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0062 | 46 |
| HLA-identical sibling | 2.633 | 0.316 | 21.95 | .371 | 104 | |
| Well-matched URD | 10.214 | 1.37 | 76.172 | .0234 | 119 | |
| Partially matched URD | 11.374 | 1.458 | 88.731 | .0204 | 45 | |
| CB | 8.129 | 1.11 | 59.526 | .0391 | 254 | |
| Race | ||||||
| White | 1.00 | — | — | — | .045 | 360 |
| Black | 2.087 | 1.206 | 3.61 | .0086 | 79 | |
| Others | 0.963 | 0.47 | 1.972 | .9183 | 75 | |
| Data missing | 0.834 | 0.33 | 2.109 | .7008 | 54 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .016 | 272 |
| ALL | 1.75 | 1.11 | 2.76 | .016 | 296 | |
| cGVHD | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | <.0001 | 46 |
| HLA-identical sibling | 2.932 | 1.079 | 7.966 | .0349 | 521 | |
| Well-matched URD | 4.508 | 1.668 | 12.181 | .003 | 659 | |
| Partially matched URD | 5.108 | 1.862 | 14.01 | .0015 | 212 | |
| CB | 3.362 | 1.233 | 9.166 | .0178 | 408 | |
| Ethnicity | .0004 | |||||
| Hispanic | 1.00 | — | — | — | 568 | |
| Non-Hispanic | 0.657 | 0.53 | 0.814 | .0001 | 1200 | |
| Data missing | 0.627 | 0.377 | 1.041 | .071 | 78 | |
| Race | ||||||
| White | 1.00 | — | — | — | <.0001 | 1340 |
| Black | 2.115 | 1.567 | 2.856 | <.0001 | 145 | |
| Others | 1.633 | 1.21 | 2.204 | .0013 | 159 | |
| Data missing | 0.863 | 0.615 | 1.211 | .3938 | 202 | |
| Relapse | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .1447 | 46 |
| HLA-identical sibling | 1.794 | 0.84 | 3.835 | .1313 | 526 | |
| Well-matched URD | 1.519 | 0.712 | 3.241 | .28 | 665 | |
| Partially matched URD | 1.261 | 0.565 | 2.814 | .5706 | 216 | |
| CB | 1.738 | 0.808 | 3.737 | .157 | 412 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | <.0001 | 876 |
| ALL | 0.626 | 0.519 | 0.755 | <.0001 | 989 | |
| TRM | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | <.0001 | 46 |
| HLA-identical sibling | 0.749 | 0.263 | 2.135 | .5885 | 526 | |
| Well-matched URD | 1.223 | 0.441 | 3.393 | .6995 | 665 | |
| Partially matched URD | 2.93 | 1.047 | 8.2 | .0406 | 216 | |
| CB | 1.743 | 0.629 | 4.831 | .2855 | 412 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .0223 | 569 |
| Non-Hispanic | 0.658 | 0.483 | 0.897 | .008 | 1215 | |
| Data missing | 0.563 | 0.243 | 1.302 | .1791 | 81 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0155 | 876 |
| ALL | 1.465 | 1.075 | 1.995 | .0155 | 989 | |
| KPS | ||||||
| ≥90% | 1.00 | — | — | — | <.0001 | 1606 |
| <90% | 2.039 | 1.44 | 2.885 | <.0001 | 259 | |
| MRD− KIR cohort and MRD− CIBMTR cohort | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0016 | 42 |
| HLA-identical sibling | 2.125 | 0.789 | 5.725 | .1359 | 74 | |
| Well-matched URD | 3.87 | 1.516 | 9.879 | .0047 | 86 | |
| Partially matched URD | 2.826 | 0.925 | 8.64 | .0684 | 27 | |
| CB | 4.485 | 1.812 | 11.099 | .0012 | 177 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .004 | 42 |
| HLA-identical sibling | 1.817 | 0.189 | 17.477 | .6052 | 74 | |
| Well-matched URD | 12.588 | 1.688 | 93.848 | .0135 | 86 | |
| Partially matched URD | 7.675 | 0.856 | 68.855 | .0687 | 27 | |
| CB | 7.345 | 0.993 | 54.355 | .0509 | 177 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0213 | 188 |
| ALL | 1.93 | 1.103 | 3.379 | .0213 | 218 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0002 | 42 |
| HLA-identical sibling | 2.525 | 0.922 | 6.919 | .0716 | 374 | |
| Well-matched URD | 4.224 | 1.556 | 11.469 | .0047 | 471 | |
| Partially matched URD | 4.617 | 1.66 | 12.844 | .0034 | 147 | |
| CB | 3.19 | 1.161 | 8.764 | .0245 | 296 | |
| Ethnicity | .0013 | |||||
| Hispanic | 1.00 | — | — | — | 401 | |
| Non-Hispanic | 0.647 | 0.499 | 0.839 | .001 | 874 | |
| Data missing | 0.455 | 0.229 | 0.905 | .0247 | 55 | |
| Race | ||||||
| White | 1.00 | — | — | — | .0001 | 965 |
| Black | 2.059 | 1.432 | 2.96 | <.0001 | 104 | |
| Others | 1.737 | 1.205 | 2.503 | .0031 | 108 | |
| Data missing | 1.015 | 0.692 | 1.487 | .9405 | 153 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0604 | 42 |
| HLA-identical sibling | 2.642 | 0.971 | 7.191 | .0572 | 378 | |
| Well-matched URD | 2.219 | 0.816 | 6.033 | .1183 | 478 | |
| Partially matched URD | 1.499 | 0.514 | 4.37 | .4588 | 149 | |
| CB | 2.506 | 0.914 | 6.869 | .0741 | 298 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | <.0001 | 609 |
| ALL | 0.554 | 0.438 | 0.7 | <.0001 | 736 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | <.0001 | 42 |
| HLA-identical sibling | 0.575 | 0.193 | 1.71 | .3195 | 378 | |
| Well-matched URD | 1.022 | 0.363 | 2.873 | .9677 | 478 | |
| Partially matched URD | 3.105 | 1.097 | 8.794 | .0329 | 149 | |
| CB | 1.545 | 0.548 | 4.356 | .4104 | 298 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0029 | 609 |
| ALL | 1.771 | 1.216 | 2.58 | .0029 | 736 | |
| KPS | ||||||
| ≥90% | 1.00 | — | — | — | .0003 | 1164 |
| <90% | 2.141 | 1.419 | 3.231 | .0003 | 181 |
| Outcome . | HR . | 95% CI, lower limit . | 95% CI, upper limit . | P . | Overall P . | n . |
|---|---|---|---|---|---|---|
| KIR cohort and CIBMTR cohort | ||||||
| aGVHD 2-4 | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0001 | 46 |
| HLA-identical sibling | 2.186 | 0.834 | 5.729 | .1117 | 104 | |
| Well-matched URD | 4.093 | 1.626 | 10.302 | .0028 | 119 | |
| Partially matched URD | 4.587 | 1.713 | 12.286 | .0024 | 45 | |
| CB | 4.907 | 2.00 | 12.039 | .0005 | 254 | |
| aGVHD 3-4 | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0062 | 46 |
| HLA-identical sibling | 2.633 | 0.316 | 21.95 | .371 | 104 | |
| Well-matched URD | 10.214 | 1.37 | 76.172 | .0234 | 119 | |
| Partially matched URD | 11.374 | 1.458 | 88.731 | .0204 | 45 | |
| CB | 8.129 | 1.11 | 59.526 | .0391 | 254 | |
| Race | ||||||
| White | 1.00 | — | — | — | .045 | 360 |
| Black | 2.087 | 1.206 | 3.61 | .0086 | 79 | |
| Others | 0.963 | 0.47 | 1.972 | .9183 | 75 | |
| Data missing | 0.834 | 0.33 | 2.109 | .7008 | 54 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .016 | 272 |
| ALL | 1.75 | 1.11 | 2.76 | .016 | 296 | |
| cGVHD | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | <.0001 | 46 |
| HLA-identical sibling | 2.932 | 1.079 | 7.966 | .0349 | 521 | |
| Well-matched URD | 4.508 | 1.668 | 12.181 | .003 | 659 | |
| Partially matched URD | 5.108 | 1.862 | 14.01 | .0015 | 212 | |
| CB | 3.362 | 1.233 | 9.166 | .0178 | 408 | |
| Ethnicity | .0004 | |||||
| Hispanic | 1.00 | — | — | — | 568 | |
| Non-Hispanic | 0.657 | 0.53 | 0.814 | .0001 | 1200 | |
| Data missing | 0.627 | 0.377 | 1.041 | .071 | 78 | |
| Race | ||||||
| White | 1.00 | — | — | — | <.0001 | 1340 |
| Black | 2.115 | 1.567 | 2.856 | <.0001 | 145 | |
| Others | 1.633 | 1.21 | 2.204 | .0013 | 159 | |
| Data missing | 0.863 | 0.615 | 1.211 | .3938 | 202 | |
| Relapse | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .1447 | 46 |
| HLA-identical sibling | 1.794 | 0.84 | 3.835 | .1313 | 526 | |
| Well-matched URD | 1.519 | 0.712 | 3.241 | .28 | 665 | |
| Partially matched URD | 1.261 | 0.565 | 2.814 | .5706 | 216 | |
| CB | 1.738 | 0.808 | 3.737 | .157 | 412 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | <.0001 | 876 |
| ALL | 0.626 | 0.519 | 0.755 | <.0001 | 989 | |
| TRM | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | <.0001 | 46 |
| HLA-identical sibling | 0.749 | 0.263 | 2.135 | .5885 | 526 | |
| Well-matched URD | 1.223 | 0.441 | 3.393 | .6995 | 665 | |
| Partially matched URD | 2.93 | 1.047 | 8.2 | .0406 | 216 | |
| CB | 1.743 | 0.629 | 4.831 | .2855 | 412 | |
| Ethnicity | ||||||
| Hispanic | 1.00 | — | — | — | .0223 | 569 |
| Non-Hispanic | 0.658 | 0.483 | 0.897 | .008 | 1215 | |
| Data missing | 0.563 | 0.243 | 1.302 | .1791 | 81 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0155 | 876 |
| ALL | 1.465 | 1.075 | 1.995 | .0155 | 989 | |
| KPS | ||||||
| ≥90% | 1.00 | — | — | — | <.0001 | 1606 |
| <90% | 2.039 | 1.44 | 2.885 | <.0001 | 259 | |
| MRD− KIR cohort and MRD− CIBMTR cohort | ||||||
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0016 | 42 |
| HLA-identical sibling | 2.125 | 0.789 | 5.725 | .1359 | 74 | |
| Well-matched URD | 3.87 | 1.516 | 9.879 | .0047 | 86 | |
| Partially matched URD | 2.826 | 0.925 | 8.64 | .0684 | 27 | |
| CB | 4.485 | 1.812 | 11.099 | .0012 | 177 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .004 | 42 |
| HLA-identical sibling | 1.817 | 0.189 | 17.477 | .6052 | 74 | |
| Well-matched URD | 12.588 | 1.688 | 93.848 | .0135 | 86 | |
| Partially matched URD | 7.675 | 0.856 | 68.855 | .0687 | 27 | |
| CB | 7.345 | 0.993 | 54.355 | .0509 | 177 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0213 | 188 |
| ALL | 1.93 | 1.103 | 3.379 | .0213 | 218 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0002 | 42 |
| HLA-identical sibling | 2.525 | 0.922 | 6.919 | .0716 | 374 | |
| Well-matched URD | 4.224 | 1.556 | 11.469 | .0047 | 471 | |
| Partially matched URD | 4.617 | 1.66 | 12.844 | .0034 | 147 | |
| CB | 3.19 | 1.161 | 8.764 | .0245 | 296 | |
| Ethnicity | .0013 | |||||
| Hispanic | 1.00 | — | — | — | 401 | |
| Non-Hispanic | 0.647 | 0.499 | 0.839 | .001 | 874 | |
| Data missing | 0.455 | 0.229 | 0.905 | .0247 | 55 | |
| Race | ||||||
| White | 1.00 | — | — | — | .0001 | 965 |
| Black | 2.059 | 1.432 | 2.96 | <.0001 | 104 | |
| Others | 1.737 | 1.205 | 2.503 | .0031 | 108 | |
| Data missing | 1.015 | 0.692 | 1.487 | .9405 | 153 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | .0604 | 42 |
| HLA-identical sibling | 2.642 | 0.971 | 7.191 | .0572 | 378 | |
| Well-matched URD | 2.219 | 0.816 | 6.033 | .1183 | 478 | |
| Partially matched URD | 1.499 | 0.514 | 4.37 | .4588 | 149 | |
| CB | 2.506 | 0.914 | 6.869 | .0741 | 298 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | <.0001 | 609 |
| ALL | 0.554 | 0.438 | 0.7 | <.0001 | 736 | |
| Donor type | ||||||
| Haploidentical | 1.00 | — | — | — | <.0001 | 42 |
| HLA-identical sibling | 0.575 | 0.193 | 1.71 | .3195 | 378 | |
| Well-matched URD | 1.022 | 0.363 | 2.873 | .9677 | 478 | |
| Partially matched URD | 3.105 | 1.097 | 8.794 | .0329 | 149 | |
| CB | 1.545 | 0.548 | 4.356 | .4104 | 298 | |
| Disease | ||||||
| AML | 1.00 | — | — | — | .0029 | 609 |
| ALL | 1.771 | 1.216 | 2.58 | .0029 | 736 | |
| KPS | ||||||
| ≥90% | 1.00 | — | — | — | .0003 | 1164 |
| <90% | 2.141 | 1.419 | 3.231 | .0003 | 181 |
Shown are data for factors associated with GVHD, relapse, and TRM (in patients with ALL or AML). Bold P-values indicate statistically significant results. KPS, Karnofsky, performance score.
Comparative adjusted statistics for the study cohorts. TRM (A), relapse (B), acute GVHD grades 2 to 4 (C), and chronic GVHD (D) for all phase 2 and CIBMTR comparator patients. Adjusted TRM (E), relapse (F), aGVHD grades 2 to 4 (G), and cGVHD (H) for all phase 2 and CIBMTR comparator patients who were MRD-negative at the time of HCT.
Comparative adjusted statistics for the study cohorts. TRM (A), relapse (B), acute GVHD grades 2 to 4 (C), and chronic GVHD (D) for all phase 2 and CIBMTR comparator patients. Adjusted TRM (E), relapse (F), aGVHD grades 2 to 4 (G), and cGVHD (H) for all phase 2 and CIBMTR comparator patients who were MRD-negative at the time of HCT.
Other notable independent risk factors for aGVHD (grades 3-4) include Black race (P = .0086) and ALL (P = .016). For cGVHD, risks included Hispanic ethnicity (P = .0004) and Black (P < .0001) or other race (P = .0013). For relapse, the other independent risk was having AML (P < .0001). For TRM, other risks included Hispanic ethnicity (P = .008), having ALL (P = .016), or having a low Karnofsky performance score (P < .0001).
Analysis of KIR favorability in a population of haploidentical transplant candidates in 2 models
We analyzed 294 pediatric patients and 646 possible donors (2.2 donors/patient; range, 1-8; standard deviation; 0.975; a superset of the 51 cases included in this report) to determine the likelihood of identifying a KIR-favorable donor by our definition. A total of 196 of 294 (66.7%) patients had ≥1 absent ligand and 188 of 196 (96%) of those had a favorable ligand-mismatched donor identified (Table 4). We evaluated the KIR haplotype B content of the potential donors and found that 193 of 646 (29.9%) had a favorable B content score9 of ≥2 (Table 5). However, when all 646 potential donors were considered in the context of the 294 patients, just under half (142 of 294; 48.3%) of the patients had at least 1 potential donor with a Cooley B-content score of ≥2, because multiple donors were screened for a given patient (Table 6). Notably, 236 of 294 (80.3%) had either a potential donor with a favorable ligand mismatch or high B content (matching our eligibility criteria), and 91 of 294 (31.0%) of patients had both favorable mismatch(es) and high B content (Table 4).
Results of KIR analysis for ligand mismatch and B content of 646 donors screened for 294 pediatric and adolescent and young adult recipients
| . | Patients screened n (%) . | Missing at least 1 ligand n (%) . | Missing C1 ligand n (%) . | Missing C2 ligand n (%) . | Missing Bw4 ligand n (%) . |
|---|---|---|---|---|---|
| Possible mismatches (absent ligand in recipient) | 294 (100) | 196 (66.7) | 51/196 (26.0) | 111/196 (56.6) | 74/196 (37.8) |
| Recipients with missing ligands who had donors available | — | 188/196 (95.9) | 49/51 (96.1) | 107/111 (96.4) | 68/74 (91.9) |
| Recipients with ≥ 1 mismatched donor at a given ligand | — | 188/294 (63.9) | 49/294 (16.7) | 107/294 (36.4) | 68/294 (23.1) |
| Recipients with a donor with ligand mismatch and Cooley score ≥2 | 91/294 (31.0) | — | — | — | — |
| Recipients with a donor that had ligand mismatch or Cooley score ≥2 or both | 236/294 (80.3) | — | — | — | — |
| . | Patients screened n (%) . | Missing at least 1 ligand n (%) . | Missing C1 ligand n (%) . | Missing C2 ligand n (%) . | Missing Bw4 ligand n (%) . |
|---|---|---|---|---|---|
| Possible mismatches (absent ligand in recipient) | 294 (100) | 196 (66.7) | 51/196 (26.0) | 111/196 (56.6) | 74/196 (37.8) |
| Recipients with missing ligands who had donors available | — | 188/196 (95.9) | 49/51 (96.1) | 107/111 (96.4) | 68/74 (91.9) |
| Recipients with ≥ 1 mismatched donor at a given ligand | — | 188/294 (63.9) | 49/294 (16.7) | 107/294 (36.4) | 68/294 (23.1) |
| Recipients with a donor with ligand mismatch and Cooley score ≥2 | 91/294 (31.0) | — | — | — | — |
| Recipients with a donor that had ligand mismatch or Cooley score ≥2 or both | 236/294 (80.3) | — | — | — | — |
Number of potential donors with a particular B-content score from the screening cohort
| B-content score . | Donors, n (%) . |
|---|---|
| 0 | 213 (33.0) |
| 1 | 240 (37.2) |
| 2 | 145 (22.4) |
| 3 | 36 (5.6) |
| 4 | 12 (1.8) |
| Total | 646 (100) |
| B-content score . | Donors, n (%) . |
|---|---|
| 0 | 213 (33.0) |
| 1 | 240 (37.2) |
| 2 | 145 (22.4) |
| 3 | 36 (5.6) |
| 4 | 12 (1.8) |
| Total | 646 (100) |
Number of patients from the screening cohort that have a potential donor with minimum B-content score
| Minimum B-content score for potential donor . | Patients, n (%) . |
|---|---|
| ≥ 0 | 294 (100) |
| ≥ 1 | 246 (83.6) |
| ≥ 2 | 142 (48.3) |
| ≥ 3 | 41 (14.1) |
| 4 | 11 (4.8) |
| Minimum B-content score for potential donor . | Patients, n (%) . |
|---|---|
| ≥ 0 | 294 (100) |
| ≥ 1 | 246 (83.6) |
| ≥ 2 | 142 (48.3) |
| ≥ 3 | 41 (14.1) |
| 4 | 11 (4.8) |
Screening cohort n = 294; potential donors n = 646.
Discussion
Although outcomes for hematological malignancies in pediatrics have improved over time,21,22 allogeneic HCT continues to play an important role in salvage of high-risk and relapsed patients. Treatment with HCT after obtaining remission after first relapse of high-risk B-ALL and T-ALL and continues to be a standard.23-25 Chimeric antigen receptor (CAR) T-cell therapies have greatly improved outcomes in patients with B-ALL who have multiple relapses, but the therapy will fail in more than half of patients, leading eventually to a transplant,26 and some argue that patients achieving remission with CAR T-cell therapy could benefit from consolidative HCT, either for all patients who have not had a previous HCT27 or for patients with characteristics that put them at high risk of relapse.28,29 AML outcomes have shown, at best, modest improvement in the past few decades, and HCT for high-risk and relapsed disease is standard.30 With a continued need for allogeneic HCT in children, URDs are often challenging to find, especially for minority patients,31 and COVID restrictions have placed a strain on donor acquisition, often forcing the use of PBSCs, which have been shown to lead to inferior outcomes in pediatric transplantation.32,33 With these concerns in mind, if haploidentical HCT approaches could be shown to be superior or, at minimum, equivalent to URD approaches, it would be of major benefit to the pediatric HCT field. Our study addresses this need and is remarkable in that we achieved outstanding results, even with the preponderance of our patients being Hispanic or Black, demonstrating that this approach can be used for diverse populations.
In this prospective, multicenter trial we demonstrated that using an approach of KIR-favorable TCR-αβ+CD3+/CD19+–depleted haploidentical HCT, a 1-year DFS of ∼80% could be realized and that DFS and OS were similar to CIBMTR outcomes when other cell sources were used. Furthermore, moderate and severe aGVHD, cGVHD, and TRM outcomes were clearly superior with our approach, and relapse risk was similar. The marked decrease in GVHD with maintenance of good outcomes was most likely the result of early licensed NK cell and/or γδ T-cell activity. Our regimens involve administering rATG more distal to the graft infusion (starting at day −12), compared with other published TCR αβ+CD3+/CD19+–depleted approaches,34 allowing less exposure of the infused graft to further depletion by rATG.35 Our approach also uses no posttransplant immune suppression and results in rapid engraftment (median, day +14), which simplifies care and facilitates adding post-HCT cellular therapies. It is notable that patients with pre-HCT by flow cytometry <0.01% had 2-year DFS and OS survival of 79% and 82%, respectively.
A challenge with haploidentical HCT has been high rates of rejection, and this has resulted in the use of intense approaches including the addition of thiotepa or melphalan to TBI or busulfan36 (in our study, traditional MAC options were TBI/thio/cy and bu/thio/cy). In this trial, rejection was rare (7.8%), occurring mostly in patients at risk for rejection through MDS or prolonged periods (2-3 months) without chemotherapy who received our rATG/flu/thio/mel approach. Although all of our rejections were rescued with salvage HCT, for patients at high risk for rejection, clinicians could consider use of our total lymphoid irradiation–based RTC regimen or more traditional myeloablative approaches. Our data were striking, however, in that they indicate that, for most patients, more intense MAC approaches may be unnecessary and possibly detrimental, as a subanalysis of patients receiving RTC vs traditional MAC approaches on our study showed superior DFS and OS with the RTC approaches. This trial did not have a sufficient number of patients with MDS to draw conclusions regarding preparative approaches for the MDS population. Another provocative finding in this study is that outcomes of patients with ALL treated with TBI vs non-TBI approaches were very similar (16 TBI-conditioned patients: DFS, 81%; 1 NRM, 2 relapse; 14 non-TBI–treated patients: DFS, 86%; 1 NRM, 1 relapse). These results differ from the recent affirmation by Peters et al23 of the superiority of TBI compared with treosulfan or busulfan when using matched sibling donors and URDs, but because our sample was small, a higher number of patients would be needed to confirm whether TBI could be omitted with this KIR-favorable haploidentical approach in MRD-negative patients with ALL, as studies addressing this issue to date have not been designed to answer this question.36,37
With the outstanding outcomes noted using our approach in a multicenter trial setting, the question arises of how this approach using TCR αβ+CD3+/CD19+–depleted KIR-favorable haploidentical donors compares with haploidentical HCT with posttransplant cy. Studies in children and younger patients treated with MAC approaches have shown EFS ranging from 55% to 79% with OS of 72% to 85%.38-40 Rates of acute GVHD have been comparable, but rates of cGVHD have varied by stem cell source (higher with PBSC use) and have in general been higher than we report herein. Our TCR αβ+CD3+/CD19+–depletion approach is designed to use PBSCs, and generally allows high doses of CD34+ cells to be given, facilitating rapid engraftment with low rates of acute and chronic GVHD. Despite a modest cost for cell processing, with early discharge and low rates of aGVHD and cGVHD, costs may be lower than those for standard approaches. A planned prospective cost analysis of our study is underway. With these issues in mind, it is unclear whether TCR αβ+CD3+/CD19+–depleted haplo-HCT is better or worse than posttransplant cy, and prospective trials are needed to answer this question. Even though our data are very promising, it is unclear whether this approach could be better than 10 of 10 or 12 of 12 matched URD HCT. A randomized, prospective trial comparing outcomes of recipients of haploidentical donors with recipients of matched URDs is the only way to answer this question. Our study was planned to lay the groundwork for such a trial and has provided key background data for Children’s Oncology Group study ASCT2031, which will randomize haploidentical approaches with fully matched URDs (approved, opening in the fall of 2022).
In summary, our prospective, multicenter trial demonstrated that the use of KIR-favorable TCR αβ+CD3+/CD19+–depleted haploidentical HCT in children with hematological malignancies leads to rates of survival that meet or exceed those of other key donor cell sources, with rates of GVHD and TRM that are lower and rates of relapse that are similar. This approach is an excellent option for patients, especially if matched sibling or URD options are not available, given our analysis showing that 80.3% of screened recipients had potential donors with a favorable ligand mismatch or high B content. This means the approach is readily available to minority populations (45% of patients on the trial were of Hispanic ethnicity and 53% were Black, Asian, or other race) who otherwise have limited donor options.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grant R01 CA181050 (NCI). Additional funding for PTCTC activities was provided by NIH, National Heart, Lung, and Blood Institute grant UG1HL069254 and a Johnny Crisstopher Children’s Charitable Foundation St. Baldrick’s Consortium Grant. Funding support for this article was provided by the NCI (R01 CA181050).
Authorship
Contribution: M.A.P., W.L., H.A.-A., D.M., and K.W.A., conceived and designed the study; M.A.P., N.J.B., N.L., E.A., A.F., M.S.C., J.-A,T., S.C., C.L.K., J.L.D., D.M., C.C.D., and H.A.-A. provided study material or patients; M.A.P., K.W.A., N.J.B., N.L., E.A., A.F., J.-A,T., S.C., C.L.K., J.L.D., D.M., C.C.D., and H.A.-A. collected and assembled the data; M.A.P., K.W.A., D.M., C.C.D., and H.A.-A. analyzed and interpreted the data; M.A.P., K.W.A., D.M., C.C.D., and H.A.-A. wrote the manuscript; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: M.A.P. has served on advisory boards for Novartis, Mesoblast, Equillium, Medexus, and Vertex; has engaged in educational activities for Novartis; and has received study support from Miltenyi (not for this trial) and Adaptive. W.L. is an employee of Miltenyi. C.C.D. has been a consultant to and served on advisory boards of Jazz Pharmaceuticals, Alexion Inc, and Omeros Corporation. J.L.D. receives royalties from Omixon. D.M. is a consultant to, owns stock options in, and receives royalties from Omixon. S.C. has served on advisory boards of AbbVie, Viacord, and Alexion. H.A.-A. received study support from Adaptive. The remaining authors declare no competing financial interests.
Correspondence: Michael A. Pulsipher, Huntsman Cancer Institute, University of Utah, 2000 Circle of Hope Dr, Room 3515, Salt Lake City, UT 84112; e-mail: michael.pulsipher@hci.utah.edu.
References
Author notes
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date. Proposals for access should be sent to michael.pulsipher@hci.utah.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
C.C.D. and H.A.-A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal