Key Points
Abnormal metaphase cytogenetics is a predictor of VTE within 12 months in newly diagnosed myeloma.
The PRISM score can identify patients at a high risk of VTE in clinical practice and for designing thromboprophylaxis trials in myeloma.
Abstract
Although venous thromboembolism (VTE) is an important treatment and disease-related complication in myeloma, a validated risk prediction model including disease-specific variables such as cytogenetics or tumor burden is lacking. The aim of this study was to develop a new risk prediction model for VTE in the context of modern antimyeloma therapy. All consecutive patients diagnosed at the Cleveland Clinic between 2008 and 2018 and with available data on baseline candidate risk factors constituted the derivation cohort. The primary outcome was VTE (deep venous thrombosis/pulmonary embolism) within 1 year of treatment initiation. A multivariable model was used, and weights were derived from subdistribution hazard ratios to construct a risk score. The model was validated both by internal bootstrap validation and in an external validation cohort. The derivation cohort consisted of 783 patients. A 5-component risk prediction tool, named the PRISM score, was developed, including the following variables: prior VTE, prior surgery, immunomodulatory drug use, abnormal metaphase cytogenetics, and Black race. The c-statistic of the model was 0.622 (95% confidence interval [CI], 0.567-0.674). The model stratified patients into low, intermediate, and high risk, with 12-month cumulative VTE incidence of 2.7%, 10.8%, and 36.5%, respectively. Risk of VTE increased significantly with increasing score in both the derivation and the external validation data sets, with a subdistribution hazard ratio per 1-point increase of 1.28 (95% CI, 1.19-1.39; P < .001) and 1.23 (95% CI, 1.07-1.41; P = .004) respectively. Although the PRISM score can guide clinicians in identifying patients at a high risk of VTE, additional external validation is necessary for incorporation into routine clinical practice.
Introduction
Venous thromboembolism (VTE) in multiple myeloma is an important disease-related complication and a treatment-related toxicity of immunomodulatory drugs (IMiDs).1-3 A large population-based study from Sweden, predominantly before the IMiD era, reported a significantly higher risk of VTE in patients with myeloma compared with matched control subjects, with the risk being highest in the first year after diagnosis.2 Subsequently, seminal studies on first-generation IMiD thalidomide showed a signal of high VTE risk, especially when given in combination with high-dose dexamethasone or doxorubicin.4-6 High VTE risk was also shown with the next-in-class IMiDs lenalidomide and pomalidomide.7,8 This led to the incorporation of routine VTE prophylaxis in myeloma, with the risk stratification based on expert consensus guidelines from the International Myeloma Working Group (IMWG), which were later adopted by the National Comprehensive Cancer Network (NCCN).9,10 Based on this risk prediction tool, low-dose aspirin is administered to patients at low risk of VTE, and prophylactic low-molecular-weight heparin is recommended for patients at high risk of VTE.9 However, attempts at externally validating the IMWG/NCCN risk prediction tool have been unsuccessful.11 Furthermore, the cumulative incidence of VTE in the first year after diagnosis remains substantial despite incorporation of IMWG/NCCN thromboprophylaxis guidelines into clinical trials and routine practice.12-14
Recently, 2 new risk prediction models have been derived and externally validated to estimate the risk of VTE in newly diagnosed myeloma, namely the SAVED and IMPEDE-VTE scores.11,15 However, there are several limitations of these models. First, disease-related variables such as tumor burden and cytogenetics were not taken into consideration in either model because they were not available in population databases (SEER [Surveillance, Epidemiology, and End Results]-Medicare and Veterans Health Administration, respectively). Second, the IMPEDE-VTE score included patients on therapeutic anticoagulation (eg, warfarin). Hence, it is of limited value to clinicians while deciding which patients may warrant aggressive thromboprophylaxis when initiating therapy. Third, both the SAVED and IMPEDE-VTE scores included a substantial number of patients who received >160 mg of dexamethasone per treatment cycle (22%-24%); this is not the current practice based on the E4A03 trial, which found a higher mortality with high-dose dexamethasone compared with low-dose dexamethasone.16 Fourth, ∼8% of patients in the IMPEDE-VTE data set received doxorubicin, which is rarely currently used as initial therapy in myeloma. Finally, the SEER-Medicare database used to derive the SAVED score only included patients aged >65 years, and the Veterans Health Administration database included predominantly older men.
Because VTE remains one of the most common adverse events with antimyeloma therapy, a risk prediction model in the context of modern antimyeloma therapy can guide clinicians to personalize the intensity of thromboprophylaxis based on the VTE and bleeding risk of individual patients. Hence, we aimed to develop and externally validate a risk prediction model for VTE in the first year after treatment initiation for patients with newly diagnosed myeloma.
Methods
Selection of derivation and validation cohorts
The study design was a retrospective cohort study, with the derivation cohort selected from the institutional database at the Cleveland Clinic. All consecutively diagnosed and treated patients with multiple myeloma at the Cleveland Clinic between January 2008 and December 2018 were included for analysis. Patients with an unknown treatment start date or inadequate follow-up until 12 months from treatment initiation were excluded. In addition, the following patients were excluded before performing univariable analysis for predictors of VTE: (1) patients with a VTE event within 6 months before treatment initiation; (2) patients on therapeutic anticoagulation for any indication; and (3) patients on more than one antithrombotic agent for prophylaxis or treatment (eg, patients on aspirin and clopidogrel).
For external validation of the risk score, all consecutive patients diagnosed and treated at the Columbia University Irving Medical Center (CUIMC) Multiple Myeloma and Amyloidosis Program between January 2012 and January 2020 were included for analysis after applying the exclusion criteria as noted in the derivation cohort selection. Patients with unknown data on components of the risk prediction score derived from the original cohort were also excluded.
The institutional review boards of both the Cleveland Clinic and CUIMC approved the study. The TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis) checklist17 is provided in supplemental Table 1 (available on the Blood website).
Assessment of outcome and risk factors
We manually extracted clinical and laboratory data from electronic medical records, including VTE events. The primary VTE outcome was defined as a deep venous thrombosis (DVT) or pulmonary embolism (PE) within 12 months of treatment initiation. Data on candidate risk factors were captured at baseline before treatment initiation. Candidate risk factors included all variables in the IMWG/NCCN guidelines9 as well as previously published SAVED11 and IMPEDE-VTE15 scores. In addition, variables that were considered clinically relevant but absent in prior scores/guidelines were also included after discussion with the senior author (A.A.K.). A total of 51 variables were included for univariable analysis (supplemental Table 2). Data on individual components of the risk prediction model derived from the derivation cohort were abstracted by manual chart review for the external validation data set. Blind assessment of outcomes or predictors was not performed because this was a retrospective cohort study.
Statistical analysis
The sample size of the study was based on the total number of eligible patients in the aforementioned time periods for the derivation and external validation cohorts. The incidence of VTE in the first 12 months after treatment initiation was estimated with cumulative incidence using the Fine and Gray method. Patients who were alive beyond 12 months with no evidence of VTE in the first 12 months were censored at the 12-month time point for estimation of cumulative VTE incidence. Univariable risk factors for VTE were assessed with Fine and Gray regression and summarized as the subdistribution hazard ratio (sHR), 95% confidence interval (CI), and P value (supplemental Table 1). Some variables had “0 events,” in which one group did not have any VTE event. For such variables, an HR cannot be calculated, and the Gray test P value is reported instead. Subsequently, stepwise selection with a variable entry criterion of P < .20 and a variable retention criterion of P < .05 was used to identify significant predictors of VTE and construct the multivariable model used to derive the risk prediction score. Patients with missing data on significant variables were excluded from the multivariate model. The multivariable model was then used to assign points to each variable based on their sHR. The reference group for each variable has sHR = 1, which was assigned a score of 0. Hence, we subtracted 1 from sHR, followed by rounding of sHR-1 to the nearest 0.5, and subsequently multiplying by 2 to make points into whole numbers. Points for each variable were summed to obtain the risk score. The sHR for a 1-point increase in score as well as the c-statistic were calculated.
After development of the risk score, recursive partitioning analysis with a log-rank splitting method was used to categorize patients into 3 risk groups. Subsequently, internal bootstrap validation was performed, and the risk score was assessed relative to VTE via the c-statistic. External validation of the risk prediction model was performed by using the CUIMC database, and results are reported as sHR per 1-point increase in score, c-statistic, and cumulative incidence of VTE in the 3 risk groups. The eligibility criteria for the external validation cohort were the same as those for the derivation cohort. We then performed calibration of the risk prediction model to estimate 12-month no-VTE survival.
For investigating the impact of VTE within 12 months of treatment initiation and overall survival (OS), landmark analysis was done at the 6- and 12-month time points. Patients with less than landmark months of follow-up were excluded, and the remaining patients were categorized as to whether they experienced VTE by the landmark time. Subsequently, OS from the landmark time point was compared between groups by using the Kaplan-Meier method.
Results
Patient characteristics
A total of 1029 consecutive patients with multiple myeloma, who were diagnosed and treated at the Cleveland Clinic between 1 January 2008, and 31 December 2018, were identified; 934 of these patients met the inclusion criteria. The median age at diagnosis was 63 years (range, 22-94 years). The study population was 55% male, and racial makeup constituted 80% White, 19% Black, and 1% other races. A total of 35% had International Staging System stage 3 disease at diagnosis, 19% had abnormal metaphase cytogenetics, and 24% had high-risk cytogenetics on fluorescence in situ hybridization (FISH). A history of VTE or arterial thromboembolism was present in 6% and 12% of patients, respectively. A total of 4% had a pelvic, femur, or hip fracture and 10% had a surgery (excluding minimally invasive procedures such as kyphoplasty or vertebroplasty) within 90 days before treatment initiation. Among 72 patients with prior surgery within 90 days, the majority had orthopedic/spine surgery (n = 60), followed by abdominal surgery (n = 5), neurosurgery (n = 4), cardiac surgery (n = 2), and thoracic surgery (n = 1). IV immunoglobulin or erythropoietin was administered as part of supportive care in 1.4% and 3.2% of patients. The most common induction regimen used was bortezomib-lenalidomide-dexamethasone (41%), with the most commonly used dexamethasone dose per cycle across regimens being 120 to 160 mg (76%). Low-dose aspirin was the most common thromboprophylaxis used (55%); approximately one-third of patients received no thromboprophylactic agent. The clinical and demographic characteristics of the entire study cohort (N = 934) and the cohort used to investigate risk factors for VTE (n = 783) are presented in Table 1.
Clinical and demographic characteristics of study cohort
| Variable . | Evaluable patients for VTE risk prediction model (n = 783) . |
|---|---|
| Age at diagnosis (median, range), y | 63 (22-91) |
| Male, % | 55.2 |
| Black/African American, % | 20.1 |
| ISS stage, no. (% of evaluable patients) | |
| I | 234 (34) |
| II | 232 (33.7) |
| III | 223 (32.4) |
| Percent BMPCs, median (range) | 50 (1-100) |
| Abnormal metaphase cytogenetics, % | 18.1 |
| High-risk FISH cytogenetics, %∗ | 23.0 |
| LDH >UNL, % | 26.7 |
| History of VTE, % | 1.5† |
| BMI, % | |
| Underweight (<18.5 kg/m2) | 1.6 |
| Normal (18.5-24.9 kg/m2) | 23.8 |
| Overweight (25-29.9 kg/m2) | 36.9 |
| Obese I (30-34.9 kg/m2) | 25.3 |
| Obese II (35-39.9 kg/m2) | 7.6 |
| Obese III (≥40 kg/m2) | 4.8 |
| Central venous catheter, % | 7.4 |
| Cardiac disease at baseline, %‡ | 13.5 |
| Type 2 diabetes mellitus, % | 15.2 |
| Chronic kidney disease, % | 10.7 |
| Pelvic, femur, or hip fracture within 90 d, % | 4.4 |
| Surgery (excluding minimally invasive kyphoplasty or vertebroplasty), % | 9.2 |
| Immobilization within 90 d, %§ | 47.0 |
| Erythropoietin use, % | 2.9 |
| History of clotting disorder, % | 0.3 |
| History of autoimmune disease, % | 5.2 |
| Hyperviscosity syndrome at diagnosis, % | 1.2 |
| Dexamethasone dose per cycle | |
| None | 0.5 |
| >0 to <120 mg | 17.4 |
| 120 to 160 mg | 76.2 |
| >160 mg | 5.9 |
| Doxorubicin use, % | 0.4 |
| Multiagent cytotoxic chemotherapy, % | 0.1 |
| Current/former smoker, % | 42.3 |
| IVIG use, % | 1.2 |
| IMiD use in frontline regimen, % | 65.4 |
| Thromboprophylaxis regimen, % | |
| None | 36.7 |
| ASA | 59.5 |
| LMWH, prophylactic | 3.8 |
| LMWH, therapeutic | 0 |
| Warfarin | 0 |
| Induction regimen, % | |
| VRd | 40.7 |
| CyBorD | 10.7 |
| VD | 22.0 |
| RD | 19.5 |
| Other | 7.0 |
| Variable . | Evaluable patients for VTE risk prediction model (n = 783) . |
|---|---|
| Age at diagnosis (median, range), y | 63 (22-91) |
| Male, % | 55.2 |
| Black/African American, % | 20.1 |
| ISS stage, no. (% of evaluable patients) | |
| I | 234 (34) |
| II | 232 (33.7) |
| III | 223 (32.4) |
| Percent BMPCs, median (range) | 50 (1-100) |
| Abnormal metaphase cytogenetics, % | 18.1 |
| High-risk FISH cytogenetics, %∗ | 23.0 |
| LDH >UNL, % | 26.7 |
| History of VTE, % | 1.5† |
| BMI, % | |
| Underweight (<18.5 kg/m2) | 1.6 |
| Normal (18.5-24.9 kg/m2) | 23.8 |
| Overweight (25-29.9 kg/m2) | 36.9 |
| Obese I (30-34.9 kg/m2) | 25.3 |
| Obese II (35-39.9 kg/m2) | 7.6 |
| Obese III (≥40 kg/m2) | 4.8 |
| Central venous catheter, % | 7.4 |
| Cardiac disease at baseline, %‡ | 13.5 |
| Type 2 diabetes mellitus, % | 15.2 |
| Chronic kidney disease, % | 10.7 |
| Pelvic, femur, or hip fracture within 90 d, % | 4.4 |
| Surgery (excluding minimally invasive kyphoplasty or vertebroplasty), % | 9.2 |
| Immobilization within 90 d, %§ | 47.0 |
| Erythropoietin use, % | 2.9 |
| History of clotting disorder, % | 0.3 |
| History of autoimmune disease, % | 5.2 |
| Hyperviscosity syndrome at diagnosis, % | 1.2 |
| Dexamethasone dose per cycle | |
| None | 0.5 |
| >0 to <120 mg | 17.4 |
| 120 to 160 mg | 76.2 |
| >160 mg | 5.9 |
| Doxorubicin use, % | 0.4 |
| Multiagent cytotoxic chemotherapy, % | 0.1 |
| Current/former smoker, % | 42.3 |
| IVIG use, % | 1.2 |
| IMiD use in frontline regimen, % | 65.4 |
| Thromboprophylaxis regimen, % | |
| None | 36.7 |
| ASA | 59.5 |
| LMWH, prophylactic | 3.8 |
| LMWH, therapeutic | 0 |
| Warfarin | 0 |
| Induction regimen, % | |
| VRd | 40.7 |
| CyBorD | 10.7 |
| VD | 22.0 |
| RD | 19.5 |
| Other | 7.0 |
ASA, acetylsalicylic acid; BMPC, bone marrow plasma cells; CyBorD, bortezomib, cyclophosphamide, and dexamethasone; IVIG, IV immunoglobulin; ISS, International Staging System; LDH, lactate dehydrogenase; UNL, upper normal limit; VRd, bortezomib-lenalidomide-dexamethasone; RD, lenalidomide-dexamethasone; VD, bortezomib-dexamethasone.
High-risk on FISH was defined as presence of t(4;14), t(14;16), t(14;20), and/or del(17p).
Patients with VTE within 6 months before treatment initiation were excluded in this group.
Cardiac disease included congestive heart failure, myocardial infarction, and clinically significant arrhythmias.
“Immobilization” was defined as any episode of hospitalization exceeding 24 hours or bed-bound status secondary to paralysis or hemiplegia in the window of 90 days before treatment initiation.
Incidence of VTE
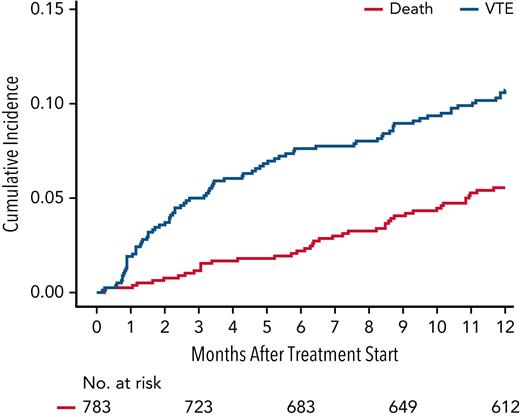
VTE in the first 12 months after treatment initiation occurred in 105 of 934 patients, with a median time to VTE of 3.2 months (95% CI, 0.2-12). The cumulative incidence of VTE at 6 months and 12 months was 8.2% (95% CI, 6.6-10.1) and 11.5% (95% CI, 9.5-13.6), respectively. The Kaplan-Meier curve for cumulative incidence of VTE is displayed in Figure 1. Among 105 patients with VTE, 85 (81%) had DVT, 16 (15%) had PE, and 6 (4%) had both DVT and PE. The most common site for DVT was lower extremity (88%). The best hematologic response at the time of VTE occurrence was ≥ partial response (PR) in 78% and ≥ very good partial response (VGPR) in 34% of patients. Disease progression at the time of VTE occurrence was observed in 9% of patients. The most common thromboprophylaxis at the time of VTE occurrence was aspirin (60.2%), followed by prophylactic LMWH (5.4%). Twenty-nine percent of patients were on no thromboprophylaxis at time of VTE.
Cumulative incidence of VTE within 12 months of treatment initiation for newly diagnosed multiple myeloma with death as a competing risk. The 6- and 12-month cumulative incidence of VTE was 8.2% (95% CI, 6.6-10.1) and 11.5% (95% CI, 9.5-13.6), respectively.
Cumulative incidence of VTE within 12 months of treatment initiation for newly diagnosed multiple myeloma with death as a competing risk. The 6- and 12-month cumulative incidence of VTE was 8.2% (95% CI, 6.6-10.1) and 11.5% (95% CI, 9.5-13.6), respectively.
Derivation of risk prediction model (PRISM score)
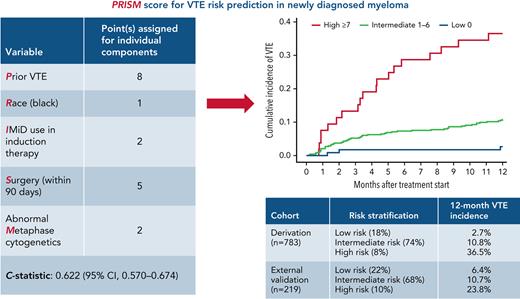
The derivation cohort consisted of 783 patients; univariable analysis was performed in these patients to identify predictors of VTE within 12 months of treatment initiation. The flowchart for patient selection is presented in supplemental Figure 1. The results of univariate analysis for risk factors of VTE within our derivation cohort are given in supplemental Table 2. Subsequently, a multivariable model was constructed that generated 5 independent predictors of VTE within the first 12 months of treatment initiation (Table 2). In decreasing order of strength of association, the factors were prior VTE (sHR, 5.06; 95% CI, 1.89-13.5), surgery within 90 days before treatment initiation (sHR, 3.44; 95% CI, 1.96-6.02), IMiD use in induction therapy (sHR, 2.17; 95% CI, 1.24-3.80), abnormal metaphase cytogenetics (sHR, 2.1; 95% CI, 1.24-3.56), and Black race (sHR, 1.71; 95% CI, 1.03-2.83). Subsequently, the multivariable model was used to assign points based on sHRs for each significant risk factor, and a risk score was developed, named PRISM. The theoretical score range was 0 to 18 in the derivation cohort. The actual distribution of the score is shown in supplemental Table 3. Regarding the risk score, sHR was 1.28 (95% CI, 1.19-1.39; P < .001) per 1-point increase in the score, with a c-statistic of 0.622 (95% CI, 0.567-0.674).
Derivation of PRISM score (multivariable Cox regression analysis)
| Variable . | Derivation cohort (Cleveland Clinic) . | ||
|---|---|---|---|
| sHR (95% CI) . | P . | Point(s)∗ . | |
| Prior history of VTE† | 5.06 (1.89-13.5) | .001 | 8 |
| Race (Black vs others) | 1.71 (1.03-2.83) | .037 | 1 |
| IMiD use in induction therapy | 2.17 (1.24-3.80) | .006 | 2 |
| Surgery (within 90 d) | 3.44 (1.96-6.02) | <.001 | 5 |
| Abnormal Metaphase cytogenetics | 2.10 (1.24-3.56) | .006 | 2 |
| Risk stratification | Low-risk: score of 0 | ||
| Intermediate-risk: score of 1-6 | |||
| High-risk: score of ≥7 | |||
| c-statistic: 0.622 (0.570-0.674) | |||
| Variable . | Derivation cohort (Cleveland Clinic) . | ||
|---|---|---|---|
| sHR (95% CI) . | P . | Point(s)∗ . | |
| Prior history of VTE† | 5.06 (1.89-13.5) | .001 | 8 |
| Race (Black vs others) | 1.71 (1.03-2.83) | .037 | 1 |
| IMiD use in induction therapy | 2.17 (1.24-3.80) | .006 | 2 |
| Surgery (within 90 d) | 3.44 (1.96-6.02) | <.001 | 5 |
| Abnormal Metaphase cytogenetics | 2.10 (1.24-3.56) | .006 | 2 |
| Risk stratification | Low-risk: score of 0 | ||
| Intermediate-risk: score of 1-6 | |||
| High-risk: score of ≥7 | |||
| c-statistic: 0.622 (0.570-0.674) | |||
Points were assigned based on HR: 1 was subtracted from each sHR, followed by rounding of “sHR-1” to the nearest 0.5, followed by multiplication by 2 to convert into a whole number.
History of VTE refers to VTE at any time before 6 months from the date of treatment initiation. Patients with VTE within 6 months before treatment initiation were excluded.
We subsequently categorized patients into 3 risk groups based on risk scores. Patients with a score of 0 were “low risk,” scores of 1 to 6 were “intermediate risk,” and scores ≥7 were “high risk.” The cumulative incidence of VTE at 12 months in low-risk, intermediate-risk, and high-risk patients was 2.7% (95% CI, 0.7-7.0), 10.8% (95% CI, 8.2-13.8), and 36.5% (95% CI, 23.6-49.6), respectively. The Kaplan-Meier curve for cumulative incidence of VTE in 3 risk groups is shown in Figure 2. Internal bootstrap validation was then performed, with 1000 bootstrap samples of size 651 that were selected with replacement from the 651 patients used to develop the multivariable model in the derivation cohort. This validation showed a median c-statistic of 0.62 with an interquartile range of 0.60 to 0.64.
Cumulative incidence of VTE within 12 months of treatment initiation stratified by risk groups derived from the PRISM score. The 12-month cumulative incidence of VTE in low-risk, intermediate-risk, and high-risk patients was 2.7% (95% CI, 0.7-7.0), 10.8% (95% CI, 8.2-13.8), and 36.5% (95% CI, 23.6-49.6), respectively.
Cumulative incidence of VTE within 12 months of treatment initiation stratified by risk groups derived from the PRISM score. The 12-month cumulative incidence of VTE in low-risk, intermediate-risk, and high-risk patients was 2.7% (95% CI, 0.7-7.0), 10.8% (95% CI, 8.2-13.8), and 36.5% (95% CI, 23.6-49.6), respectively.
External validation of the PRISM score
External validation of the PRISM score was performed in a database of patients from the CUIMC Multiple Myeloma and Amyloidosis Program. A total of 257 consecutive patients diagnosed and treated at CUIMC between January 2012 and January 2020 and with available baseline data on VTE predictors in the PRISM score were included for analysis. After applying the inclusion/exclusion criteria for the PRISM score, 38 patients were excluded due to the following: (1) therapeutic anticoagulation (n = 24); (2) multiple antithrombotic agents (n = 5); (3) VTE within 6 months before treatment initiation (n = 4); and (4) unknown metaphase cytogenetics (n = 4). This led to a total of 219 patients with 23 VTE events within 1 year of treatment initiation. The key baseline clinical and demographic characteristics of the validation cohort and comparison with the derivation cohort are presented in supplemental Table 4. Compared with the derivation cohort, median age at treatment initiation was significantly higher in the validation cohort (63 vs 67 years, respectively; P < .001), and IMiD use as a part of induction therapy was lower in the validation cohort (65% vs 45%; P < .001). Furthermore, a significantly higher proportion of patients had abnormal metaphase cytogenetics in the validation cohort compared with the derivation cohort (36% vs 18%; P < .001).
The 12-month cumulative incidence of VTE in the validation cohort was 11.0% (95% CI, 7.2-15.6). The sHR per 1-point score increase in the validation cohort was 1.23 (95% CI, 1.07-1.41; P = .004), with a c-statistic of 0.587 (95% CI, 0.492-0.682). The direction and magnitude of effect size for risk predictors were similar for all variables except Black race, which did not predict for VTE in the external validation cohort. Notably, abnormal metaphase cytogenetics was a significant predictor of VTE in the validation cohort as well (sHR for abnormal vs normal metaphase cytogenetics at baseline, 3.8; 95% CI, 1.5-9.6; P = .005). The cumulative VTE incidence across risk groups in the derivation and validation cohorts is summarized in Table 3. The sHRs between PRISM risk groups in the derivation and validation cohorts are summarized in supplemental Table 5. Subsequently, we performed calibration of the model in the validation cohort. Using the Kaplan-Meier method, the observed and predicted 12-month no-VTE survival rates were 0.887 and 0.888, respectively.
Cumulative incidence of VTE at 12 months
| PRISM risk category . | Derivation cohort (95% CI) . | External validation cohort (95% CI) . |
|---|---|---|
| Low (0) | 2.7% (0.7-7.0) | 6.4% (1.6-15.9) |
| Intermediate (1-6) | 10.8% (8.2-13.8) | 10.7% (6.2-16.4) |
| High (≥7) | 36.5% (23.6-49.6) | 23.8% (8.3-43.6) |
| PRISM risk category . | Derivation cohort (95% CI) . | External validation cohort (95% CI) . |
|---|---|---|
| Low (0) | 2.7% (0.7-7.0) | 6.4% (1.6-15.9) |
| Intermediate (1-6) | 10.8% (8.2-13.8) | 10.7% (6.2-16.4) |
| High (≥7) | 36.5% (23.6-49.6) | 23.8% (8.3-43.6) |
Impact of VTE on survival
The impact of VTE within 12 months of diagnosis on OS was assessed in the entire cohort (N = 934). The median follow-up of surviving patients was 37 months (range, 1-134 months). The 5-year OS estimate for patients with and without VTE at the 6-month landmark analysis was 52% and 60%, respectively (P = .23). The 5-year OS estimate for patients with and without VTE at the 12-month landmark analysis was 58% and 63% (P = .40).
Discussion
The risk of VTE in the first year after diagnosis of multiple myeloma remains substantial at ∼11%. Using 2 independent institutional databases, we have successfully derived and externally validated a risk prediction model for VTE in myeloma in the context of modern antimyeloma therapy. The new risk prediction model, named PRISM, contains 5 variables, which are intuitive and universally available at diagnosis: prior history of VTE, Black race, IMiD use in induction therapy, surgery within 90 days, and abnormal metaphase cytogenetics. The PRISM score was able to differentiate patients into 3 groups based on their risk of developing VTE within 12 months of treatment initiation. Notably, onset of VTE within 12 months did not have any impact on OS in the landmark analysis.
To our knowledge, the PRISM score is the first risk prediction tool in myeloma to include abnormal metaphase cytogenetics as a significant predictor of VTE, with the direction as well as magnitude of effect size comparable in both the derivation and external validation data sets. Although cytogenetics tested by interphase FISH (iFISH) on CD-138–selected cells is positive for abnormalities in almost all patients with myeloma, metaphase cytogenetics is abnormal in ∼20% to 30% of patients.18,19 Abnormal metaphase cytogenetics is an indicator of aggressive biology and proliferation of plasma cells independent of the bone marrow stroma.19,20 Furthermore, it also predicts poor survival, irrespective of the type of chromosomal abnormality found.18,21 Conversely, only high-risk cytogenetic abnormalities on iFISH predict for poor survival.19,20 Notably, in the TT2 (Total Therapy 2) trial, which randomized patients to treatment with thalidomide vs no thalidomide in the context of cytotoxic chemotherapy and tandem autotransplant, abnormal metaphase cytogenetics was associated with a significantly higher risk of VTE on multivariable analysis, with an HR of 2.14 (95% CI, 1.15-3.98; P = .016).22 Indeed, increased cytogenetic risk on karyotype or metaphase cytogenetics has also been shown to predict VTE risk in other hematologic malignancies such as acute myeloid leukemia.23 High-risk cytogenetic abnormalities on iFISH did not predict VTE in our study, which is in line with the findings from another study on ∼500 patients with myeloma.24 These data are consistent with our findings that abnormal metaphase cytogenetics is a significant predictor of VTE in myeloma.
The risk factors associated with VTE that were common to both SAVED and IMPEDE-VTE scores were prior VTE and high-dose dexamethasone (>160 mg/cycle), whereas the only factor associated with decreased risk was Asian/Pacific Islander race.11,15 Among these risk factors, prior VTE was a significant predictor of subsequent VTE within 12 months of treatment initiation in the PRISM score. The proportion of patients receiving high-dose dexamethasone and of Asian/Pacific Islander race was low in our data set (6% and <1%, respectively), which may have led to a lack of identification of these factors as a significant predictor of VTE. However, the antimyeloma therapies used in our data set better represent current clinical practice. Other risk factors in the PRISM score that were shared with prior risk prediction tools were surgery and use of IMiDs.11 Patients with myeloma are often diagnosed with a pathologic facture in long bones leading to orthopedic surgical intervention. A study on ∼1.65 million patients undergoing 76 surgical procedures found that the presence of malignancy and history of VTE were associated with a higher odds of subsequent VTE within 3 months’ postsurgery.25 Furthermore, compared with patients without myeloma, surgical treatment of hip fracture in myeloma patients is associated with a greater incidence of early complications, including in-hospital pneumonia, sepsis, infection of the surgical site, and acute renal failure that may lead to prolonged immobilization.26 Although pelvic/femur/hip fracture, prior surgery, and prior immobilization were all associated with a significant risk of VTE on univariable analysis in the PRISM derivation data set, only prior surgery was significant on multivariable analysis. Notably, Black race was a significant predictor of VTE in the PRISM derivation data set. In the derivation cohort for the SAVED score, the HR for VTE in Black subjects (compared with White subjects) was 1.33 (P = .13) on univariable analysis.11 Notably, an analysis of 51 149 individuals enrolled in 3 prospective trials found that Black patients had a significantly higher incidence of VTE compared with White patients in the ARIC (Atherosclerotic Risk in Communities) study (HR, 1.81; 95% CI, 1.20-2.73) but not in the CHS (Cardiovascular Health Study) study (HR, 1.20; 95% CI, 0.96-1.54).27 Furthermore, in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, there was a significant interaction between geographical region and race, with Black patients in the Southeast having a significantly higher risk of VTE compared with the rest of the nation (HR, 1.63; 95% CI, 1.08-2.48). Larger data sets of myeloma patients from different geographical regions are needed to definitively identify the association of Black race with VTE in myeloma.
The current study did not show an adverse impact of VTE within 12 months of treatment initiation on OS in the landmark analysis. There are conflicting data on the association between VTE and survival in myeloma. Although large population-based studies from Sweden (1987-2005) and the Veterans Health Administration (1999-2014) databases have shown a negative impact of VTE on OS, with HRs ranging from 1.7 to 2.9,28,29 data from the Myeloma IX/XI trials and the MM-009/MM-010 trials did not show any adverse impact of VTE on OS.12 Furthermore, in the Swedish study, although VTE was associated with worse OS, a 6-month landmark analysis did not show an adverse impact of early VTE on OS.28 It is plausible that many of the early VTE events are partially due to an adverse effect of IMiDs and not related to aggressive tumor biology, and hence are not predictive of worse survival. Apart from survival, important late effects that can occur after VTE include post-phlebitis syndrome, chronic thromboembolic pulmonary hypertension, and recurrent VTE,30,31 which makes the prevention of VTE an important component of supportive care in myeloma.
The current study has limitations. First, the total number of VTE events was low, especially in the external validation data set. Second, we only accounted for candidate risk factors at baseline and not time-varying risk factors. Because patients with newly diagnosed myeloma can experience VTE-provoking events in the first few months (eg, fractures requiring surgery), future studies should investigate the role of incorporating time-varying VTE risk factors. Third, we have not performed prospective validation of our risk prediction tool, which is considered the gold standard. Furthermore, the discrimination of SAVED and IMPEDE-VTE models in their external validation data sets was similar to slightly superior compared with PRISM external validation (c-statistics of 0.60, 0.64, and 0.59, respectively).11,15 However, the strength of our model is the availability of individual patient-level data in both derivation and external validation data sets, including data on disease-specific factors that were not accounted for in derivation of prior risk scores. Although our model calibrated well in the external validation data set, we acknowledge that the discrimination was lower compared with that of the derivation cohort, and the VTE estimate in the high-risk group decreased from 36.5% in the derivation cohort to 23.8% in the validation cohort. Future studies should further validate its performance in larger external data sets.
In conclusion, PRISM is the first validated risk prediction tool including patient-, disease-, and treatment-specific factors that was developed in the context of modern antimyeloma therapy and a patient population that is representative of demographic characteristics of the United States. However, external validation in larger data sets as well as prospective validation, which is the gold standard, will be required before routine incorporation of the PRISM score into clinical practice. In the interim, it can guide clinicians in assessing VTE risk and risk/benefit profile of thromboprophylaxis strategies. Given the substantial risk of VTE in intermediate- and high-risk patients, these patients may benefit from direct oral anticoagulants during the first year after treatment initiation, which has been shown to lower the risk of VTE in myeloma in nonrandomized studies.32-34 Future studies should test direct oral anticoagulants in a randomized fashion in trials enriched for patients at intermediate and high risk of VTE.
Authorship
Contribution: R.C. and A.A.K. designed the study; R.C. abstracted clinical data, wrote the first draft of the manuscript, and approved the final version; A.A.K. provided critical feedback in study design, statistical analysis, manuscript writing, and approved the final version of the manuscript; L.R. and W.W. performed statistical analysis, provided critical feedback, and approved the final version of the manuscript; J.V., B.M.F., C.J.S., and F.A. performed clinical management, provided critical feedback in the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rajshekhar Chakraborty, Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center, Herbert Irving Pavilion, Floor 9, 161 Fort Washington Ave, New York, NY 10032; e-mail: rc3360@cumc.columbia.edu.
References
Author notes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal