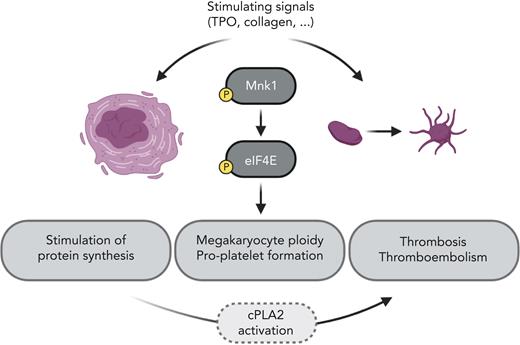
In this issue of Blood, Manne et al1 explore for the first time the role of MAPK-interacting kinase 1 (Mnk1) in megakaryocytes and platelets (see figure). They show that Mnk1 regulates protein synthesis by phosphorylation of eukaryotic translation initiation factor 4E (eIF4E) and also affects megakaryocyte (MK) ploidy and platelet production. This study helps to deepen our knowledge of activation pathways in MKs and platelets that affect platelet function and thrombosis. Mnk1 inhibitors are currently under development and in clinical testing, and these results may have clinical implications.
Activation of megakaryocytes and platelets stimulates Mnk1 and eIF4E phosphorylation and increases messenger RNA translation, megakaryocyte ploidy, and proplatelet formation, as well as thrombosis, most likely via the activation of cPLA2. TPO, thrombopoietin. Illustration created with the help of Tony Gutschner using BioRender.com.
Activation of megakaryocytes and platelets stimulates Mnk1 and eIF4E phosphorylation and increases messenger RNA translation, megakaryocyte ploidy, and proplatelet formation, as well as thrombosis, most likely via the activation of cPLA2. TPO, thrombopoietin. Illustration created with the help of Tony Gutschner using BioRender.com.
Platelets are important players in hemostasis. The life cycle of platelets begins with proplatelet biogenesis from MKs in response to various stimuli.2 MKs themselves develop from hematopoietic stem cells. In response to thrombopoietin, they grow and increase their genetic information via endomitosis.2 Although it has long been thought that platelets are incapable of regulating gene expression, we now know that the key regulators of protein synthesis (ie, eIF4E and eIF2α) are present and abundant in platelets.3 The cap-binding protein eIF4E is phosphorylated by Mnk at Ser209 in vitro and in vivo.4,5 Mnks are ubiquitously expressed and represent a class of enzymes that are activated downstream of the p38 mitogen-activated protein kinase (MAPK) and MAPK/extracellular signal-regulated kinase (MAPK/ERK) pathways in response to mitogenic stimuli and stress.4,6 The existence of MAPKs in platelets was described more than 20 years ago.7 In addition, the expression of Mnk1 in platelets has been reported.8 However, the precise role of Mnks in MKs and platelets and their functional implications have not been fully explored.
In their study, Manne et al performed elegant and robust in vitro and in vivo experiments to define the role of Mnk1 in megakaryocytes and platelets. Pharmacological and genetic manipulations of Mnk1 expression confirmed its role in phosphorylating eIF4E upon stimulation of MKs or platelets, thereby regulating protein synthesis. Polysome profiling showed that inhibition of Mnk1 by CGP 57380 resulted in a higher monosome fraction and a lower polysome peak, and [35S]methionine incorporation assays confirmed reduced de novo protein synthesis. In addition, the absence of Mnk1 in MKs resulted in reduced MK ploidy and proplatelet formation in vitro. In vivo, platelet production was significantly lower in Mnk1 knockout (KO) mice without changing platelet half-life. To find out which messenger RNAs were translationally regulated by Mnk1, Manne et al used ribosome footprint profiling of megakaryocytes from wild-type and Mnk1 KO mice and identified that Mnk1 regulates the translation of PLA2G4A, which encodes the cytosolic phospholipase A2 (cPLA2). cPLA2 hydrolyzes polyunsaturated fatty acids, such as arachidonic acid from platelet membranes that subsequently are metabolized by platelet oxygenases,9 stimulating production of thromboxane A2.10 Indeed, they showed that cPLA2 protein expression and activation are downregulated in megakaryocytes that lack Mnk1, and thromboxane production is significantly reduced in activated platelets from Mnk1 KO mice, further confirming the importance of Mnk1 for platelet function and aggregation. Finally, to prove their hypothesis, Manne et al performed 2 independent in vivo experiments that showed that Mnk1 KO mice developed fewer and smaller thrombi and were protected from pulmonary thromboembolism-induced death.
Altogether, Manne et al have found novel important players in platelet production and function (see figure). However, some effects were modest, and it is likely that other regulators and downstream targets besides cPLA2 play a role. Certainly, future studies will be conducted to explore this question. In addition, this current study involved a germline Mnk1 KO mouse model, whereas future studies should use MK and platelet-specific KO models to further explore the function of Mnk1 in platelets and thrombosis specifically in vivo. Furthermore, it will be interesting to elucidate the role of Mnk1 in physiological and pathophysiological conditions, where platelets are considered important, such as wound healing, inflammatory processes, or cancer-associated thromboembolism, besides their role in hemostasis.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal