Key Points
LIF protects mice against GVHD by reducing the infiltration and activation of donor immune cells and protecting intestinal stem cells.
LIF decreases IL-12 levels in recipient dendritic cells and MHC-II levels on intestinal epithelia to reduce T-cell activation and GVHD-induced damage.
Abstract
Graft-versus-host disease (GVHD) remains a major complication after allogeneic hematopoietic stem cell transplantation, a widely used therapy for hematologic malignancies and blood disorders. Here, we report an unexpected role of cytokine leukemia inhibitory factor (LIF) in protecting against GVHD development. Administrating recombinant LIF protein (rLIF) protects mice from GVHD-induced tissue damage and lethality without compromising the graft-versus-leukemia activity, which is crucial to prevent tumor relapse. We found that rLIF decreases the infiltration and activation of donor immune cells and protects intestinal stem cells to ameliorate GVHD. Mechanistically, rLIF downregulates IL-12–p40 expression in recipient dendritic cells after irradiation through activating STAT1 signaling, which results in decreased major histocompatibility complex II levels on intestinal epithelial cells and decreased donor T-cell activation and infiltration. This study reveals a previously unidentified protective role of LIF for GVHD-induced tissue pathology and provides a potential effective therapeutic strategy to limit tissue pathology without compromising antileukemic efficacy.
Introduction
Allogeneic bone marrow transplantation (allo-BMT) is a potentially curative therapy for patients with hematologic malignancies.1-3 The therapeutic benefits of allo-BMT are derived from cytoreductive conditioning and a donor immune cell–mediated graft-versus-leukemia (GVL) effect.1-3 However, this treatment is often limited by the development of graft-versus-host disease (GVHD). Acute GVHD is mainly induced by the antigen disparity between the donor and recipient.1-3 The alloreactive donor T cells, activated by host antigen-presenting cells (APCs), cause damage in vital recipient organs, classically the gastrointestinal (GI) tract, skin, and liver, and initiate GVHD.1 GVHD affects up to 70% of allo-BMT patients, with 20% to 75% mortality rates.4 Limiting GHVD while maintaining GVL activity remains a critical goal in the clinic.
The GI tract is the main organ targeted by donor T cells during GVHD. Damage to the GI tract is the primary determinant of GVHD severity and lethality.5 Shortly after allo-BMT, donor T cells migrate to the GI tract, and preferentially invade the crypt intestinal stem cell (ISC) compartment, where they damage ISCs, leading to GI injury and GVHD.6 A recent study revealed that the elevation of cytokine interleukin 12 (IL-12) levels by irradiation before allo-BMT contributes to GI injury during GVHD development.5 The elevation of IL-12 induces major histocompatibility complex class II (MHC-II) expression on the surface of intestinal epithelial cells (IECs), which serve as APCs to activate donor T cells to induce GI injury and trigger GVHD.5
Leukemia inhibitory factor (LIF) was initially identified as a factor that induces the terminal differentiation of murine myeloid leukemia M1 cells (for a review, see Zhang et al7). Subsequent studies revealed that LIF plays an essential role in many physiological and pathological processes, including self-renewal of pluripotent stem cells, maternal reproduction, inflammation, and tumorigenesis in a highly cell-, tissue- and context-dependent manner.7 Recently, we found that LIF is essential in maintaining ISC number and functions to ensure the intestinal epithelial homeostasis and regeneration.8 LIF has been reported to regulate immune response and inflammation through regulating dendritic cells (DCs), regulatory T cells (Tregs), and macrophages under different conditions.9-11 Considering the roles of ISC damage and inflammation in triggering GVHD,6 we investigated the role of LIF in GVHD.
In this study, we found that LIF levels are elevated at the early stage of GVHD and serum LIF levels after allo-BMT are positively correlated with the survival of mice succumbing to GVHD. Importantly, administrating recombinant LIF protein (rLIF) protects mice from GVHD-induced tissue damage and lethality without compromising the GVL activity. rLIF decreases the infiltration and activation of donor immune cells and protects ISCs to ameliorate GVHD. This study unveils an important role of LIF in inflammatory immune response in the context of GVHD and provides a promising therapeutic strategy for GVHD.
Methods
Mice
C57BL/6 (H2kb), CD45.2 (H2kb), C3H/HeJ (H2kk), and BALB/c (H2kd) mice were obtained from the Jackson Laboratory. B6C3F1 (H2kb/k) mice were produced by crossing C57BL/6 mice with C3H/HeJ mice. IL-12–p40–IRES–eYFP mice were generated by Richard Locksley at UCLA.12 Conventional LIF knockout mice in C57BL/6 background were obtained from EMMA repository (EM:02619). Age- and gender-matched mice at 8 to 12 weeks of age were used for experiments in this study. Animals were assigned randomly to different treatment groups. Sample sizes were chosen based on the power calculation. For total body irradiation (TBI) treatment, mice were subjected to 11 Gy TBI with a 137Cs γ-source irradiator at a dose rate of 90 cGy/min. For rLIF treatment, mice were treated with an intraperitoneal (IP) injection of either mouse rLIF (Millipore) (30 ng/g body weight) or phosphate-buffered saline (PBS) twice a day for different durations. For recombinant IL-12 protein (rIL-12) treatment, mice were treated with an IP injection of either mouse rIL-12 (R&D Systems) (500 ng/d) or PBS once a day for 7 days (starting from 4 days before BMT until 3 days after BMT). The investigators were blinded to the group allocation during experiments and when assessing outcomes. All mouse experiments were approved by the Institutional Animal Care and Use Committee of Rutgers University.
BMT
For BMT, transplant recipients were lethally irradiated with 13 Gy TBI (split doses of 2 × 6.5 Gy with a 4-hour interval) or 11 Gy TBI (split doses of 2 × 5.5 Gy with a 4-hour interval) 1 day before BMT (D-1), followed by intravenous transplantation of 5 × 106 bone marrow (BM) cells with or without T cells (6 × 106 for B6C3F1 recipients and 8 × 106 for C57BL/6 recipients) at day 0 (D0). Mice were monitored twice a week for body weight, GVHD score, and survival.
Statistical analyses
All data were obtained from at least 3 repetitions and were expressed as mean ± standard deviation or mean ± standard error of the mean as indicated in the figure legends. The survival of mice was summarized by Kaplan-Meier plots and compared by the log-rank test using the GraphPad Prism software. The body weight loss and GVHD score curve were compared by analysis of variance using the GraphPad Prism software. All other P values were obtained by the Student t test and Welch’s correction was performed for data with big variation. P values of less than .05 were considered significant. All data points and n values reflect biological replicates.
All other methods are described in detail in the supplemental Methods.
Results
Serum LIF levels are increased upon allo-BMT and positively correlated with the lifespan of mice with GVHD
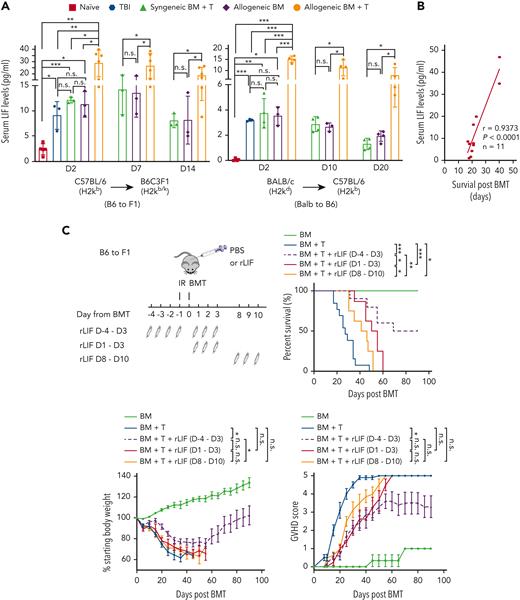
To investigate the potential role of LIF in GVHD, we measured serum LIF levels after allo-BMT in mice, including B6C3F1 (H2kb/k) mice receiving allo-BMT from C57BL/6 (H2kb) mice, an MHC-haploidentical GVHD model,13,14 and C57BL/6 (H2kb) mice receiving allo-BMT from BALB/C (H2kd) mice, an MHC-mismatched GVHD model.15 Mice were irradiated with 5.5 Gy TBI twice, followed by BMT 1 day after irradiation, denoted as day 0. Naïve mice, mice that received TBI only, BMT from syngeneic mice (syn-BMT), and BM from allogeneic mice (allo-BM) served as controls. Serum LIF levels were significantly elevated in mice at 2 days after TBI as determined by enzyme-linked immunosorbent assays (ELISAs) (Figure 1A). Although syn-BMT and allo-BM induced serum LIF levels to a similar extent as TBI did, allo-BMT induced serum LIF to much higher levels, and the elevation of serum LIF levels can still be observed at 14 days and 20 days after treatments in B6C3F1 and C57BL/6 mice, respectively (Figure 1A). LIF production in different tissues was examined at messenger RNA (mRNA) levels by quantitative real-time PCR. LIF induction was most obvious at 2 days after treatments and then gradually decreased at later time points in all tissues examined (supplemental Figure 1). TBI, syn-BMT, and allo-BM showed strong LIF induction in the colon and small intestine (SI), followed by spleen and mesenteric lymph nodes (MLNs), whereas allo-BMT showed very high LIF induction in the MLNs and spleen followed by colon and SI (supplemental Figure 1). Notably, in the majority of tissues and time points examined, allo-BMT induced much higher levels of LIF than TBI, syn-BMT, or allo-BM, which was consistent with the change observed in serum LIF levels (Figure 1A). These results suggest that both radiation and early GVHD alloreactivity contribute to LIF induction. Whereas B6C3F1 mice receiving allo-BMT developed GVHD rapidly, their serum LIF levels after allo-BMT were positively correlated with their lifespan after allo-BMT (Figure 1B), suggesting a protective role of LIF against GVHD.
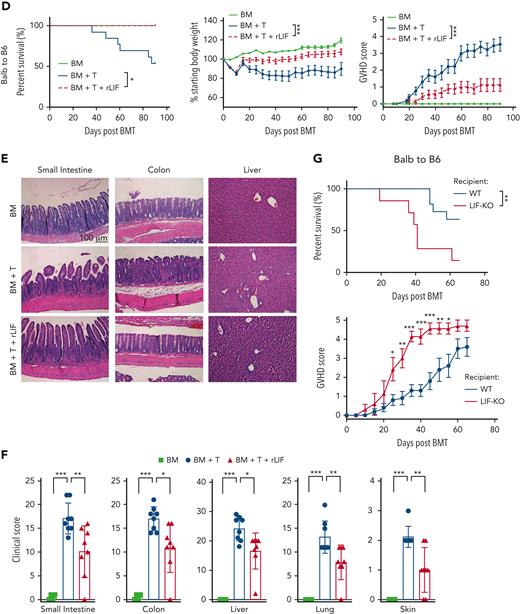
Administering rLIF ameliorates GVHD in mice. (A) Serum LIF levels in B6C3F1 (left) and C57BL/6 (right) mice at different days (D) after TBI (2 × 5.5 Gy), syngeneic BM + T, allogenic BM, and allogenic BM + T. n ≥ 3 mice/group (each dot represents a mouse). Serum LIF levels were measured by ELISAs. (B) The serum LIF levels at 2 days after allo-BMT were positively correlated with the survival length of mice after allo-BMT. B6C3F1 mice were exposed to 2 × 5.5 Gy TBI, followed by transplantation of BM and T cells from C57BL/6 mice. (C) Lethally irradiated B6C3F1 mice received BM alone (BM; n = 3), BM and T cells from C57BL/6 mice (BM + T; n = 13), or BM and T cells along with rLIF treatment (IP, 30 ng/g body weight, twice a day for the period as indicated) (BM + T + rLIF; n = 10 for D-4 to D3; n = 8 for D1 to D3 and D8 to D10). Schematic diagram of experimental procedures is shown on the upper left. Three panels shown are Kaplan-Meier survival curves (upper right), weight loss (lower left), and GVHD score (lower right) of mice after allo-BMT. (D) Lethally irradiated C57BL/6 mice received BM (n = 3), BM + T from BALB/c mice (n = 12), or BM + T + rLIF (n = 9). Mice were treated with rLIF as follows: IP, 30 ng/g body weight, twice a day for 7 days (D-4 to D3). Three panels shown are Kaplan-Meier survival curves (left), weight loss (middle), and GVHD score (right) of mice after allo-BMT. (E,F) Tissues from lethally irradiated B6C3F1 mice receiving C57BL/6 BM (n = 4), BM + T (n = 8) or BM + T + rLIF (n = 8). Tissues from these mice were collected at 14 days after allo-BMT for histopathologic analysis. (E) Representative hematoxylin and eosin–stained images of SI, colon, and liver tissues. (F) The clinical score was analyzed as described in Methods to reflect the histopathologic damage in different tissues. (G) Lethally irradiated C57BL/6 wild-type (n = 11) and LIF knockout (n = 7) mice received BM + T from BALB/c mice. Kaplan-Meier survival curves (upper) and GVHD score (lower) of mice after allo-BMT are presented. Data presented are from at least 3 independent experiments. (A,F) Data are presented as mean ± standard deviation; (C-D,G) data are presented as mean ± standard error of the mean. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, n.s.: not significant; unpaired t test with Welch’s correction (A,F), Spearman’s correlation (B), Kaplan-Meier survival analysis for survival, and analysis of variance for analysis of weight loss and GVHD score.
Administering rLIF ameliorates GVHD in mice. (A) Serum LIF levels in B6C3F1 (left) and C57BL/6 (right) mice at different days (D) after TBI (2 × 5.5 Gy), syngeneic BM + T, allogenic BM, and allogenic BM + T. n ≥ 3 mice/group (each dot represents a mouse). Serum LIF levels were measured by ELISAs. (B) The serum LIF levels at 2 days after allo-BMT were positively correlated with the survival length of mice after allo-BMT. B6C3F1 mice were exposed to 2 × 5.5 Gy TBI, followed by transplantation of BM and T cells from C57BL/6 mice. (C) Lethally irradiated B6C3F1 mice received BM alone (BM; n = 3), BM and T cells from C57BL/6 mice (BM + T; n = 13), or BM and T cells along with rLIF treatment (IP, 30 ng/g body weight, twice a day for the period as indicated) (BM + T + rLIF; n = 10 for D-4 to D3; n = 8 for D1 to D3 and D8 to D10). Schematic diagram of experimental procedures is shown on the upper left. Three panels shown are Kaplan-Meier survival curves (upper right), weight loss (lower left), and GVHD score (lower right) of mice after allo-BMT. (D) Lethally irradiated C57BL/6 mice received BM (n = 3), BM + T from BALB/c mice (n = 12), or BM + T + rLIF (n = 9). Mice were treated with rLIF as follows: IP, 30 ng/g body weight, twice a day for 7 days (D-4 to D3). Three panels shown are Kaplan-Meier survival curves (left), weight loss (middle), and GVHD score (right) of mice after allo-BMT. (E,F) Tissues from lethally irradiated B6C3F1 mice receiving C57BL/6 BM (n = 4), BM + T (n = 8) or BM + T + rLIF (n = 8). Tissues from these mice were collected at 14 days after allo-BMT for histopathologic analysis. (E) Representative hematoxylin and eosin–stained images of SI, colon, and liver tissues. (F) The clinical score was analyzed as described in Methods to reflect the histopathologic damage in different tissues. (G) Lethally irradiated C57BL/6 wild-type (n = 11) and LIF knockout (n = 7) mice received BM + T from BALB/c mice. Kaplan-Meier survival curves (upper) and GVHD score (lower) of mice after allo-BMT are presented. Data presented are from at least 3 independent experiments. (A,F) Data are presented as mean ± standard deviation; (C-D,G) data are presented as mean ± standard error of the mean. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, n.s.: not significant; unpaired t test with Welch’s correction (A,F), Spearman’s correlation (B), Kaplan-Meier survival analysis for survival, and analysis of variance for analysis of weight loss and GVHD score.
rLIF administration ameliorates GVHD in mice
We tested the role of LIF in GVHD by using the GVHD models described earlier. B6C3F1 mice receiving allo-BMT from C57BL/6 mice developed GVHD rapidly, showing severe body weight loss and increased GVHD scores (characterized by weight loss, hunching posture, skin lesions, dull fur, and diarrhea),16 and died at approximately 30 days after allo-BMT (Figure 1C). Notably, rLIF administration (IP, 30 ng/g body weight, twice a day, starting 4 days before BMT until 3 days after BMT; D-4 to D3) in B6C3F1 mice significantly ameliorated GVHD; rLIF-treated mice exhibited significantly prolonged survival, much reduced weight loss, and significantly lower GVHD scores than PBS-treated mice (Figure 1C). Donor cell chimerism reached a level of 70% to 95% at 7 days after allo-BMT in the spleen, MLN, and lamina propria (LP) tissues in B6C3F1 mice with or without rLIF treatment as examined by flow cytometry, validating the proper donor cell chimerism (supplemental Figure 2). The gating strategies of flow cytometry are shown in supplemental Figure 3. The protective effect of rLIF on GVHD was also observed when rLIF was administered from D1 to D3 from BMT or even when GVHD has developed (D8 to D10 from BMT), although to less of an extent when compared with rLIF administration from D-4 to D3 from BMT (Figure 1C). Therefore, rLIF was administered from D-4 to D3 from BMT in the following experiments. Similarly, rLIF ameliorated GVHD in C57BL/6 mice receiving allo-BMT from BALB/C mice (Figure 1D). Histopathological analysis showed that multiple organs, including the SI, colon, liver, lung, and skin, displayed pathological injuries in B6C3F1 mice at 14 days after allo-BMT (Figure 1E-F). These pathological injuries were markedly decreased in rLIF-treated mice (Figure 1E-F). The protective effects of LIF were also observed when the TBI dose was increased to 6.5 Gy twice in both GVHD models (supplemental Figure 4A-B). In C57BL/6 recipients, especially at the higher TBI dose, rLIF exhibited the most significant protection on GVHD-induced tissue injuries in SI (supplemental Figure 4C). Further, LIF deficiency in recipient mice (LIF knockout) significantly increased GVHD severity as reflected by significantly decreased survival and higher GVHD scores than wild-type recipient mice (Figure 1G). Together, these results demonstrate that rLIF ameliorates GVHD in mice.
rLIF decreases donor immune cell infiltration and inhibits systemic inflammation in recipient mice after BMT
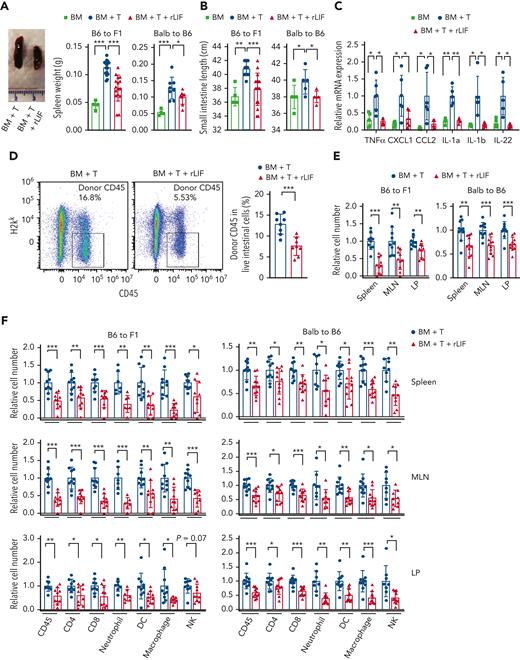
The tissue injury in GVHD is associated with inflammation, which is caused by the infiltration and activation of donor allogeneic T (allo-T) cells.1 In both GVHD models, the mice with GVHD displayed inflammation as indicated by the swollen and increased weight of spleen and increased length of SI, which is a main organ targeted by donor T cells during GVHD5,6 (Figure 2A-B). Notably, rLIF significantly decreased inflammation in both GVHD models; compared with PBS-treated mice, rLIF-treated mice displayed a much less pronounced increase of spleen weight and SI length (Figure 2A-B). In B6C3F1 mice, the mRNA levels of several proinflammatory cytokines, including TNFα, CXCL1, CCL2, IL-1a, IL-1b, and IL-22, were significantly increased in the SI at 7 days after allo-BMT as determined by quantitative real-time PCR (Figure 2C). Notably, rLIF largely abolished the increase of these cytokine levels (Figure 2C). It is worth noting that although IL-22 can display a proinflammatory effect and was reported to promote GVHD development,17,18 IL-22 was also shown to protect ISCs during inflammatory intestinal damage to regulate sensitivity to GVHD,19,20 indicating the complex role of IL-22 in GVHD. Taken together, these results suggest an essential role of LIF in suppressing systemic inflammation induced by allo-BMT to prevent GVHD.
rLIF administration decreases tissue inflammation and donor immune cell infiltration after allo-BMT. Lethally irradiated B6C3F1 and C57BL/6 mice received BM and BM + T cells from C57BL/6 and BALB/c mice, respectively, along with or without rLIF treatment. (A-B) BM+T increased spleen weight (A) and length of SI (B) in B6C3F1 mice and C57BL/6 mice at 7 days and 10 days after allo-BMT, respectively, which was largely abolished by rLIF administration. (A, left) Representative images of spleen tissues. For B6C3F1 mice: n = 4 for BM; n = 16 for both BM + T and BM + T + rLIF; for C57BL/6 mice: n ≥ 4 for BM; n ≥ 5 for both BM + T and BM + T + rLIF. (C) rLIF administration reduced the expression of majority inflammatory cytokines examined in B6C3F1 mice at 7 days after allo-BMT. Relative mRNA expression levels of TNFα, CXCL1, CCL2, IL-1a, IL-1b, and IL-22 in SI were determined by quantitative real-time PCR assays and normalized with β-actin. n ≥ 5 mice/group. (D) rLIF administration decreased the infiltration of donor CD45+ immune cells in epithelium of the intestine (EPI) from B6C3F1 mice at 7 days after allo-BMT. Representative flow cytometry images (left) and quantifications (right) show the percentage of donor CD45+ immune cells (H2kk–CD45+) in EPI from B6C3F1 mice at 7 days after allo-BMT. n = 8 mice/group. (E-F) rLIF administration decreased the donor immune cell infiltration in B6C3F1 (left) and C57BL/6 (right) mice at 7 and 10 days after allo-BMT, respectively. (E) The numbers of infiltrating donor cells in spleen, MLN, and LP tissues determined by flow cytometric assays. (F) The numbers of a set of infiltrating donor immune cells, including CD45, CD4, CD8, neutrophil, DC, macrophage and natural killer (NK) cells, in spleen (upper), MLN (middle), and LP (lower) tissues. The number of cells in mice that received BM + T without rLIF treatment was defined as 1. Gating strategies are shown in supplemental Figure 3. n ≥ 7 mice/group. Data are presented as mean ± standard deviation from at least 3 independent experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, unpaired t test with Welch’s correction.
rLIF administration decreases tissue inflammation and donor immune cell infiltration after allo-BMT. Lethally irradiated B6C3F1 and C57BL/6 mice received BM and BM + T cells from C57BL/6 and BALB/c mice, respectively, along with or without rLIF treatment. (A-B) BM+T increased spleen weight (A) and length of SI (B) in B6C3F1 mice and C57BL/6 mice at 7 days and 10 days after allo-BMT, respectively, which was largely abolished by rLIF administration. (A, left) Representative images of spleen tissues. For B6C3F1 mice: n = 4 for BM; n = 16 for both BM + T and BM + T + rLIF; for C57BL/6 mice: n ≥ 4 for BM; n ≥ 5 for both BM + T and BM + T + rLIF. (C) rLIF administration reduced the expression of majority inflammatory cytokines examined in B6C3F1 mice at 7 days after allo-BMT. Relative mRNA expression levels of TNFα, CXCL1, CCL2, IL-1a, IL-1b, and IL-22 in SI were determined by quantitative real-time PCR assays and normalized with β-actin. n ≥ 5 mice/group. (D) rLIF administration decreased the infiltration of donor CD45+ immune cells in epithelium of the intestine (EPI) from B6C3F1 mice at 7 days after allo-BMT. Representative flow cytometry images (left) and quantifications (right) show the percentage of donor CD45+ immune cells (H2kk–CD45+) in EPI from B6C3F1 mice at 7 days after allo-BMT. n = 8 mice/group. (E-F) rLIF administration decreased the donor immune cell infiltration in B6C3F1 (left) and C57BL/6 (right) mice at 7 and 10 days after allo-BMT, respectively. (E) The numbers of infiltrating donor cells in spleen, MLN, and LP tissues determined by flow cytometric assays. (F) The numbers of a set of infiltrating donor immune cells, including CD45, CD4, CD8, neutrophil, DC, macrophage and natural killer (NK) cells, in spleen (upper), MLN (middle), and LP (lower) tissues. The number of cells in mice that received BM + T without rLIF treatment was defined as 1. Gating strategies are shown in supplemental Figure 3. n ≥ 7 mice/group. Data are presented as mean ± standard deviation from at least 3 independent experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, unpaired t test with Welch’s correction.
Systemic inflammation is caused by the infiltration of donor immune cells, particularly T cells.1 We accessed the effect of rLIF on donor immune cell infiltration. The composition and number of donor immune cells were measured by flow cytometry in multiple lymphoid organs, including the spleen, MLN (an important site of immune cell activation for the SI),3 and the SI, which includes both LP (a main immune compartment of SI) and intraepithelial lymphocytes that reside within the epithelium of the intestine.21 rLIF significantly decreased the number of infiltrating donor immune cells (H2kk–CD45+) in EPI represented as the percent of cells in the total population of intestinal cells in B6C3F1 mice at 7 days after allo-BMT (Figure 2D). rLIF significantly decreased the number of infiltrating donor cells (H2kk– and H2kb– for B6C3F1 and C57BL/6 recipients, respectively), and the numbers of most types of immune cells, including CD45+ cells, CD4+ and CD8+ T cells, myeloid cells (neutrophils, DCs, and macrophages), and natural killer cells, in the spleen, MLN, and LP in both GVHD models (Figure 2E-F). This effect of rLIF on donor immune cell number was not observed in a syn-BMT C57BL/6 CD45.1 to C57BL/6 CD45.2 mouse model (supplemental Figure 5). LIF was reported to promote Tregs and inhibit T helper 17 cells, a subset of proinflammatory T cells producing IL-17, under certain conditions.11,22 We found that rLIF administration increased the percentage of Tregs in the infiltrating CD4 cells but showed no obvious effect on T helper 17 cells in the spleen, MLN, and LP in B6C3F1 mice after allo-BMT (supplemental Figure 6). Collectively, these results demonstrate that rLIF suppresses the infiltration of donor immune cells to inhibit systemic inflammation in mice after allo-BMT.
rLIF protects ISCs from GVHD-induced damage
It was reported that, upon allo-BMT, donor T cells were recruited to the recipient ISC compartment where they attack ISCs to initiate inflammation and subsequently GVHD.6 Recently, we found that LIF is essential for ISC function and protects against irradiation-induced intestinal damage.8 Here, we investigated whether the protection of ISCs by LIF contributes to its role in ameliorating GVHD. We examined the proliferation of intestinal epithelium and the number of viable ISCs in B6C3F1 mice at 2 days after allo-BMT using immunohistochemistry staining of Ki67, a cell proliferation marker, and Olfm4, an ISC marker.8 Allo-BMT decreased the number of viable crypts (defined as a crypt-like structure containing at least 5 adjacent Ki67+ cells) and the Olfm4+ viable ISCs in the SI after allo-BMT, which was significantly rescued by rLIF administration (Figure 3A-B). Interferon gamma (IFNγ), an important cytokine that induces ISC death in GVHD,23 induced the death of intestinal organoids in a dose-dependent manner, which was significantly decreased by rLIF (Figure 3C). These results demonstrate an important role of LIF in protecting the ISCs from allo-T cell–induced damage, contributing to the function of LIF in ameliorating GVHD.
rLIF administration protects ISCs from GVHD-induced damage. Administration of rLIF significantly increased the number of proliferating crypts (A) and viable ISCs (B) in B6C3F1 mice at 2 days after allo-BMT. (A-B, upper) Representative images of immunohistochemistry staining of Ki67 (A) and Olfm4 (B) in the duodenum and ileum tissues. (A-B, lower) Quantification of viable crypts per field (A) and Olfm4-positive crypts per field (B) in the duodenum and ileum of mice after allo-BMT. n = 30 fields from at least 3 mice/group. (C) IFNγ induced cell death in intestinal organoids derived from B6C3F1 mice, which was largely decreased by rLIF. Representative images of organoid growth (left). Quantification of organoid viability (right). n = 4/group. Data are presented as mean ± standard deviation. ∗∗∗P < .001, n.s.: not significant. Student t test.
rLIF administration protects ISCs from GVHD-induced damage. Administration of rLIF significantly increased the number of proliferating crypts (A) and viable ISCs (B) in B6C3F1 mice at 2 days after allo-BMT. (A-B, upper) Representative images of immunohistochemistry staining of Ki67 (A) and Olfm4 (B) in the duodenum and ileum tissues. (A-B, lower) Quantification of viable crypts per field (A) and Olfm4-positive crypts per field (B) in the duodenum and ileum of mice after allo-BMT. n = 30 fields from at least 3 mice/group. (C) IFNγ induced cell death in intestinal organoids derived from B6C3F1 mice, which was largely decreased by rLIF. Representative images of organoid growth (left). Quantification of organoid viability (right). n = 4/group. Data are presented as mean ± standard deviation. ∗∗∗P < .001, n.s.: not significant. Student t test.
rLIF inhibits donor T cell activation by suppressing MHC-II expression on IECs
APCs are essential for immune response by processing and presenting antigens to lymphocytes, including T cells, to activate these cells.24 Intriguingly, IECs can serve as APCs and play an important role in GVHD; TBI elevates MHC-II levels on IECs, which enhances donor T cell activation and leads to GVHD.5 Indeed, a significant induction of MHC-II levels on IECs was observed in B6C3F1 and C57BL/6 mice after allo-BMT as determined by flow cytometry and immunofluorescence staining assays (Figure 4A-B). Notably, rLIF suppressed the antigen-presenting function of IECs by significantly decreasing MHC-II induction on IECs after allo-BMT (Figure 4A-B). rLIF showed no obvious effect on MHC-II expression on macrophages and DCs in the spleen and MLNs (supplemental Figure 7A). IFNγ induced MHC-II expression on IECs in intestinal organoids (supplemental Figure 7B), which is consistent with previous reports.25,26 rLIF showed no obvious effect on IFNγ-induced MHC-II expression in intestinal organoids (supplemental Figure 7B), excluding the possibility that LIF directly regulates MHC-II expression on IECs. In line with the decreased MHC-II levels, rLIF greatly decreased the number of activated donor T cells (CD25+/CD69+/KLRG1+ cells27,28) (Figure 4C). These results demonstrate that LIF inhibits MHC-II expression on IECs after allo-BMT, which in turn decreases donor T cell activation.
rLIF administration inhibits the elevation of MHC-II expression on IECs and donor T cell activation after allo-BMT. (A-B) Allo-BMT increased MHC-II presentation on IECs, which was reduced by administering rLIF in mice. (A, left) Representative histograms (left) and quantifications of mean florescence intensity (MFI) (right) of MHC-II levels on IECs from B6C3F1 mice at 7 days after allo-BMT. (A, right) Representative histograms (left) and quantifications of MFI (right) of MHC-II levels on IECs from C57BL/6 mice at 10 days after allo-BMT. For B6C3F1 recipients, n = 3 for BM; n = 8 for both BM + T and BM + T + rLIF; for C57BL/6 recipients, n = 3 for BM; n = 10 for both BM + T and BM + T + rLIF. (B, upper) Representative immunofluorescence staining of MHC-II levels on IECs in the SI from C57BL/6 mice at different days after allo-BMT. (B, lower) Quantifications of MHC-II MFI. n ≥ 3 mice/group. (C) The number of donor activated T cells in the MLN (upper) and LP (lower) tissues from B6C3F1 mice at 7 days after allo-BMT with or without rLIF administration. n ≥ 8 mice/group. Data are presented as mean ± standard deviation. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, unpaired t test with Welch’s correction.
rLIF administration inhibits the elevation of MHC-II expression on IECs and donor T cell activation after allo-BMT. (A-B) Allo-BMT increased MHC-II presentation on IECs, which was reduced by administering rLIF in mice. (A, left) Representative histograms (left) and quantifications of mean florescence intensity (MFI) (right) of MHC-II levels on IECs from B6C3F1 mice at 7 days after allo-BMT. (A, right) Representative histograms (left) and quantifications of MFI (right) of MHC-II levels on IECs from C57BL/6 mice at 10 days after allo-BMT. For B6C3F1 recipients, n = 3 for BM; n = 8 for both BM + T and BM + T + rLIF; for C57BL/6 recipients, n = 3 for BM; n = 10 for both BM + T and BM + T + rLIF. (B, upper) Representative immunofluorescence staining of MHC-II levels on IECs in the SI from C57BL/6 mice at different days after allo-BMT. (B, lower) Quantifications of MHC-II MFI. n ≥ 3 mice/group. (C) The number of donor activated T cells in the MLN (upper) and LP (lower) tissues from B6C3F1 mice at 7 days after allo-BMT with or without rLIF administration. n ≥ 8 mice/group. Data are presented as mean ± standard deviation. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, unpaired t test with Welch’s correction.
rLIF suppresses IL-12 production in recipient DCs to inhibit Th1 differentiation and protect against GVHD
MLNs are crucial in regulating the immune microenvironment of the SI.3 We compared the cytokines released from MLNs after TBI between mice with and without rLIF administration. TBI (11 Gy) in C57BL/6 mice led to a decrease in many immune cell types in MLNs owing to cell death at 24 hours after TBI, which was the time to perform BMT (supplemental Figure 8A). Notably, TBI did not change DC numbers in MLNs (supplemental Figure 8A). We measured the mRNA levels of cytokines in DCs isolated from MLNs after TBI by using a nanostring myeloid innate immunity gene expression panel. TBI altered the cytokine expression patterns in DCs (supplemental Figure 8B). IL12b encoding IL-12–p40, a well-known inflammatory cytokine, stood out with obvious induction by TBI in DCs, and its induction was largely decreased by rLIF (supplemental Figure 8B). Consistently, IL-12–p40 was greatly induced by TBI in DCs, and its induction was significantly decreased by rLIF as analyzed by using a cytokine array measuring the protein levels of cytokines in DCs (Figure 5A and supplemental Figure 8C). This finding was validated by using the IL-12–p40–IRES–eYFP (IL-12–p40–YFP) mouse model, which has a knock-in allele expressing YFP from the IL-12–p40 locus and can be used to measure IL-12–p40 production in vivo.29 TBI greatly increased the percentage and number of IL-12–p40–YFP+ DCs, which were significantly decreased by rLIF (Figure 5B and supplemental Figure 8D). Consistently, TBI increased IL-12–p40 levels in MLNs and serum as measured by ELISAs, which were significantly decreased by rLIF (supplemental Figure 8E). Bioactive IL-12 (IL-12–p70), which is required for IFNγ production and T helper 1 (Th1) cell differentiation, is a 70-kDa heterodimer protein composed of 2 subunits: p35 and p40.30 Consistent with the results of IL-12–p40, TBI increased IL-12–p70 levels in MLNs, which was largely abolished by rLIF (supplemental Figure 8F). rLIF had no obvious effect on the levels of IL-23, another important cytokine for GVHD pathology that shares the IL-12–p40 subunit with IL-1231 (supplemental Figure 8G). IL-12–p40 can be produced by DCs and macrophages.30 We found that DCs but not macrophages were the predominant cell type that produces IL-12–p40 in MLNs (supplemental Figure 8D).
rLIF administration inhibits radiation-induced IL-12 production in DCs through the STAT1 signaling to protect against GVHD. (A) TBI (11 Gy) induced IL-12 production from DCs in MLNs, which was greatly reduced by rLIF administration as examined by using the cytokine panel at 24 hours after TBI in C57BL/6 mice. n = 8 mice/group. (B) TBI (11 Gy) increased percentage (left) and number (right) of IL-12+ cells in DCs in MLNs at 24 hours after TBI, which were largely decreased by rLIF administration in IL-12–p40–YFP C57BL/6 reporter mice as examined by flow cytometric assays. n ≥ 4 mice/group. The gating strategy and representative flow images are shown in supplemental Figure 8D. (C) The significantly decreased expression of T-bet in donor CD4+ T cells in MLNs from B6C3F1 mice at 7 days after allo-BMT with rLIF administration compared with mice without rLIF administration as determined by flow cytometric assays. n = 8 mice/group. (D) The induction of the expression of Th1 cytokine IFNγ by BM + T in B6C3F1 mice was largely decreased by rLIF administration as determined at 7 days after allo-BMT. Relative mRNA expression levels of IFNγ in the SI were determined by quantitative real-time PCR assays (left). Protein levels of IFNγ in the SI (middle) and serum (right) were determined by ELISAs. n ≥ 4 mice/group. (E-F) Administering rIL-12 largely abolished the protective effect of rLIF on GVHD. Lethally irradiated B6C3F1 mice that received allo-BMT from C57BL/6 mice along with or without rLIF administration were treated with rIL-12 (500 ng/d for 7 days from D-4 to D3) or PBS. (E) The spleen weight (left) and length of SI (right) in B6C3F1 mice measured at 7 days after allo-BMT. (F) MFI of MHC-II on IECs of B6C3F1 mice were determined at 7 days after allo-BMT. n ≥ 3 mice/group. (G-H) Administering rIL-12 largely abolished the inhibitory effect of rLIF on donor immune cell infiltration after allo-BMT. (G) The relative numbers of infiltrating donor cells in the spleen, MLN, and LP tissues from B6C3F1 mice at 7 days after allo-BMT. (H) The relative numbers of infiltrating donor immune cells in the spleen (top), MLN (middle), and LP (bottom) tissues from B6C3F1 mice at 7 days after allo-BMT. n ≥ 6 mice/group. (I) BMDCs were activated by IFNγ (10 ng/ml) and LPS (100 ng/ml) with or without LIF treatment (100 ng/ml) for 6 hours. The mRNA levels of IL12b in BMDCs were determined by quantitative real-time PCR assays and normalized with β-actin. n = 7/group. (J) rLIF treatment increased the phosphorylation levels of STAT1 at Tyr-701 (p-STAT1) in activated BMDC as determined by Western blot assays. (K) rLIF treatment increased the binding of STAT1 to a putative STAT1 binding site in the intron 1 of IL12b gene as determined in BMDCs by chromatin immunoprecipitation assays. (Top) The sequence and location of the putative STAT1 binding site in IL12b gene. A region containing no STAT1 binding site was included as a negative control. n = 5/group. n.d.: non-detectable. (L) Blocking the STAT1 signaling by fludarabine (2 μM) and pravastatin (2 μM), 2 small-molecule STAT1 inhibitors, largely abolished the inhibitory effect of rLIF on IL12b production in activated BMDCs. The mRNA levels of IL12b in BMDCs were determined by quantitative real-time PCR assays and normalized with β-actin. n = 3/group. Data are presented as mean ± standard deviation from 3 independent experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, n.s.: not significant; unpaired t test with Welch’s correction.
rLIF administration inhibits radiation-induced IL-12 production in DCs through the STAT1 signaling to protect against GVHD. (A) TBI (11 Gy) induced IL-12 production from DCs in MLNs, which was greatly reduced by rLIF administration as examined by using the cytokine panel at 24 hours after TBI in C57BL/6 mice. n = 8 mice/group. (B) TBI (11 Gy) increased percentage (left) and number (right) of IL-12+ cells in DCs in MLNs at 24 hours after TBI, which were largely decreased by rLIF administration in IL-12–p40–YFP C57BL/6 reporter mice as examined by flow cytometric assays. n ≥ 4 mice/group. The gating strategy and representative flow images are shown in supplemental Figure 8D. (C) The significantly decreased expression of T-bet in donor CD4+ T cells in MLNs from B6C3F1 mice at 7 days after allo-BMT with rLIF administration compared with mice without rLIF administration as determined by flow cytometric assays. n = 8 mice/group. (D) The induction of the expression of Th1 cytokine IFNγ by BM + T in B6C3F1 mice was largely decreased by rLIF administration as determined at 7 days after allo-BMT. Relative mRNA expression levels of IFNγ in the SI were determined by quantitative real-time PCR assays (left). Protein levels of IFNγ in the SI (middle) and serum (right) were determined by ELISAs. n ≥ 4 mice/group. (E-F) Administering rIL-12 largely abolished the protective effect of rLIF on GVHD. Lethally irradiated B6C3F1 mice that received allo-BMT from C57BL/6 mice along with or without rLIF administration were treated with rIL-12 (500 ng/d for 7 days from D-4 to D3) or PBS. (E) The spleen weight (left) and length of SI (right) in B6C3F1 mice measured at 7 days after allo-BMT. (F) MFI of MHC-II on IECs of B6C3F1 mice were determined at 7 days after allo-BMT. n ≥ 3 mice/group. (G-H) Administering rIL-12 largely abolished the inhibitory effect of rLIF on donor immune cell infiltration after allo-BMT. (G) The relative numbers of infiltrating donor cells in the spleen, MLN, and LP tissues from B6C3F1 mice at 7 days after allo-BMT. (H) The relative numbers of infiltrating donor immune cells in the spleen (top), MLN (middle), and LP (bottom) tissues from B6C3F1 mice at 7 days after allo-BMT. n ≥ 6 mice/group. (I) BMDCs were activated by IFNγ (10 ng/ml) and LPS (100 ng/ml) with or without LIF treatment (100 ng/ml) for 6 hours. The mRNA levels of IL12b in BMDCs were determined by quantitative real-time PCR assays and normalized with β-actin. n = 7/group. (J) rLIF treatment increased the phosphorylation levels of STAT1 at Tyr-701 (p-STAT1) in activated BMDC as determined by Western blot assays. (K) rLIF treatment increased the binding of STAT1 to a putative STAT1 binding site in the intron 1 of IL12b gene as determined in BMDCs by chromatin immunoprecipitation assays. (Top) The sequence and location of the putative STAT1 binding site in IL12b gene. A region containing no STAT1 binding site was included as a negative control. n = 5/group. n.d.: non-detectable. (L) Blocking the STAT1 signaling by fludarabine (2 μM) and pravastatin (2 μM), 2 small-molecule STAT1 inhibitors, largely abolished the inhibitory effect of rLIF on IL12b production in activated BMDCs. The mRNA levels of IL12b in BMDCs were determined by quantitative real-time PCR assays and normalized with β-actin. n = 3/group. Data are presented as mean ± standard deviation from 3 independent experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, n.s.: not significant; unpaired t test with Welch’s correction.
We examined whether the inhibitory effect of rLIF on IL-12–p40 induction by TBI suppresses donor Th1 differentiation after allo-BMT. The expression of the transcription factor T-bet in CD4+ T cells is a hallmark of Th1 differentiation.5 Notably, in B6C3F1 mice with allo-BMT, T-bet levels in donor CD4+ T cells were much lower in MLNs from rLIF-treated mice than PBS-treated mice (Figure 5C). Consistently, the mRNA levels of IFNγ, encoding a well-known Th1 cytokine,32 in SI, protein levels of IFNγ in SI and serum, and the numbers of infiltrating donor CD4+ IFNγ+ cells in MLNs were much lower in rLIF-treated mice than PBS-treated mice (Figure 5D and supplemental Figure 9A). rLIF had no obvious effect on Th1 differentiation in vitro (supplemental Figure 9B-C), excluding the possibility the LIF directly inhibits Th1 differentiation.
IL-12 was recently reported to induce MHC-II expression on IECs upon TBI.5 Here, we tested whether the inhibitory effect of LIF on TBI-induced IL-12 contributes to the inhibitory effect of LIF on the increase of MHC-II level on IECs induced by TBI and subsequent protection against GVHD. B6C3F1 mice with allo-BMT were administered with rIL-12. Intriguingly, rIL-12 largely abolished the protective effect of rLIF on GVHD, as indicated by the increased spleen weight and SI length, elevated MHC-II expression on IECs, enhanced infiltration of donor immune cells in the spleen, and MLN and LP tissues in mice receiving allo-BMT along with rLIF and rIL-12 treatments compared with mice receiving allo-BMT and rLIF treatment (Figure 5E-H). In contrast, rIL-12 exhibited a very limited effect on mice receiving allo-BMT without rLIF treatment (Figure 5E-H). These results indicate that the regulation of IL-12 levels by LIF mediates the protective effect of LIF on GVHD.
rLIF regulates IL-12 in DCs through the STAT1 signaling
To study the mechanism whereby LIF regulates IL-12 production in DCs, we used BM-derived DCs (BMDCs) as an in vitro model.33 IFNγ and lipopolysaccharides (LPS) were used to stimulate BMDCs, which mimics a radiation-induced inflammatory microenvironment. IFNγ and LPS treatment significantly increased IL12b production in BMDCs, which was largely abolished by rLIF (Figure 5I). This finding is consistent with the results from our in vivo experiments showing that rLIF decreased IL-12–p40 production from DCs after TBI (Figure 5A-B and supplemental Figure 8B-D). LIF exerts its function through selectively activating its downstream pathways in a highly tissue- and cell type–specific manner.7 We examined a panel of well-known LIF downstream pathways, including the STATs, AKT, ERK, and MAPK pathways,7 in BMDCs derived from C57BL/6 and B6C3F1 upon IFNγ and LPS stimulation. rLIF greatly enhanced STAT1 activity as reflected by the increased levels of STAT1 phosphorylation at Tyr-701, but not the total STAT1 protein levels in BMDCs treated with IFNγ and LPS (Figure 5J and supplemental Figure 10A). No clear activation of other major signaling pathways was observed in BMDCs upon rLIF treatment (Figure 5J and supplemental Figure 10A). The specificity of the antibodies was validated in supplemental Figure 10B-C. As a transcription factor, STAT1 mainly exerts its function through binding to the promoter region of its target genes to activate or repress their transcription.34,35 The signaling through STAT proteins plays complex roles in DC activation. Currently, the role of STAT1 in IFNγ and LPS-induced IL-12–p40 expression is unclear. It was reported that the STAT1 signaling inhibits CD40L-induced IL-12 production in DCs.36 Here, we investigated whether LIF regulates IL-12–p40 production in BMDCs through transcriptional regulation by STAT1. A putative STAT-binding cis-acting element37,38 was identified in the intron 1 of the mouse IL12b gene encoding IL-12–p40 (Figure 5K). Chromatin immunoprecipitation assays showed that rLIF significantly enhanced the binding of STAT1 to the regulatory region of IL12b containing the potential STAT1-binding elements in BMDCs activated by IFNγ and LPS (Figure 5K). Furthermore, blocking the STAT1 function in BMDCs by 2 small-molecule STAT1 inhibitors, fludarabine39 and pravastatin,40 greatly abolished the inhibitory effect of rLIF on IL12b expression in BMDCs, and showed a much less pronounced effect on IL12b expression in BMDCs without LIF treatment (Figure 5L). Collectively, these results suggest that LIF inhibits IL-12–p40 production in DCs upon inflammatory stimulation, mainly through the STAT1 signaling.
rLIF preserves the GVL effect
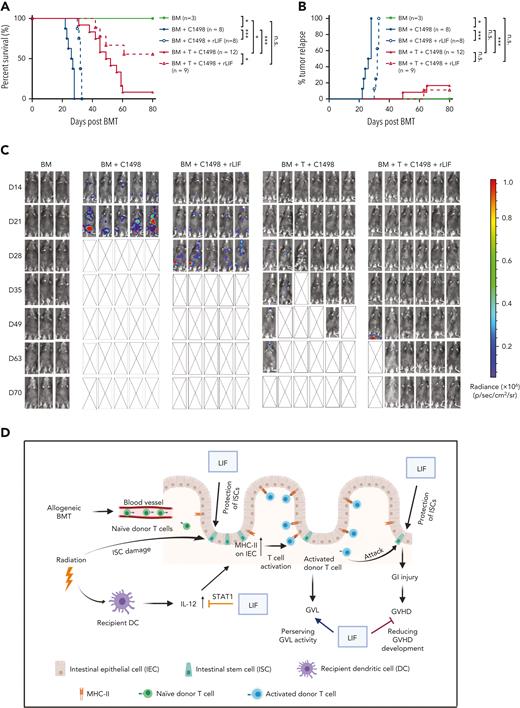
Although responsible for GVHD, allo–T cells play a key role in eliminating residual leukemia cells to prevent tumor relapse (known as GVL), which is the key to the success of BMT.2 Here, we determined whether rLIF affects GVL by using the BALB/C to C57BL/6 BMT model supplemented with C1498 murine acute myeloid leukemia cells with a luciferase reporter transduced for bioluminescence imaging tumor tracking, which is a well-established GVL model.41 The mice receiving BM and C1498 cells quickly succumbed to leukemia with strong bioluminescence imaging signals reflecting leukemia development, and all mice died by 28 days after BMT (Figure 6A-C). rLIF inhibited and delayed leukemia growth and prolonged the survival of mice receiving BM and C1498 cells by approximately 5 days (Figure 6A-C). Although the addition of allo–T cells largely prevented leukemia relapse in mice with allo-BMT, these mice had GVHD as a major cause of mortality with a median lifespan of approximately 50 days after allo-BMT (Figure 6A-C). Importantly, rLIF significantly improved the survival of mice with allo-BMT; more than 50% of mice were still alive at the end of the experiment at 80 days after allo-BMT (Figure 6A). These results indicate that rLIF administration alleviates GVHD and, at the same time, preserves the GVL activity (Figure 6D).
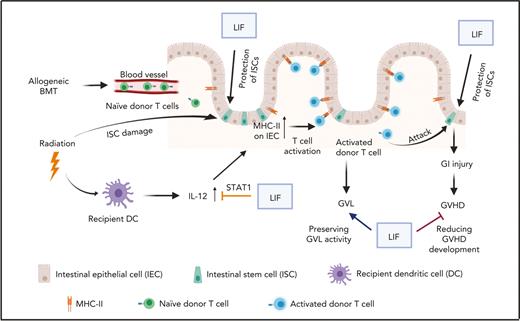
rLIF administration effectively ameliorates GVHD and preserves the GVL effect. (A-C) Lethally irradiated C57BL/6 mice were transplanted with BM with or without T cells from BALB/c mice along with luciferase-labelled C1498 mouse leukemia cells. The day of BMT was denoted as D0. Recipient mice were treated with vehicle (PBS) or rLIF (IP, 30 ng/g body weight, twice a day, from D-4 to D3). (A) Kaplan-Meier survival curve of mice. (B) Percentage of tumor relapse in mice. (C) Representative bioluminescence images of mice throughout the experiment. Kaplan-Meier survival analysis was used to compare among groups. ∗P < .05, ∗∗∗P < .001, n.s.: not significant. (D) Schematic illustration of the role of LIF in protecting against GVHD. The diagram was prepared by using BioRender software.
rLIF administration effectively ameliorates GVHD and preserves the GVL effect. (A-C) Lethally irradiated C57BL/6 mice were transplanted with BM with or without T cells from BALB/c mice along with luciferase-labelled C1498 mouse leukemia cells. The day of BMT was denoted as D0. Recipient mice were treated with vehicle (PBS) or rLIF (IP, 30 ng/g body weight, twice a day, from D-4 to D3). (A) Kaplan-Meier survival curve of mice. (B) Percentage of tumor relapse in mice. (C) Representative bioluminescence images of mice throughout the experiment. Kaplan-Meier survival analysis was used to compare among groups. ∗P < .05, ∗∗∗P < .001, n.s.: not significant. (D) Schematic illustration of the role of LIF in protecting against GVHD. The diagram was prepared by using BioRender software.
Discussion
LIF plays important roles in many physiological and pathological conditions (for a review, see Zhang et al7 or Yue et al42). However, the role of LIF in immune cell- and inflammation-caused damage in the context of GVHD remains unknown. In this study, by using preclinical GVHD mouse models, we demonstrated that serum LIF levels were significantly increased in mice undergoing allo-BMT and the serum LIF levels after allo-BMT were positively correlated with the survival of mice after allo-BMT that succumbed to GVHD. Although these results indicate the potential protecting effect of LIF on GVHD, the fact that mice receiving allo-BMT developed GVHD indicates that additional LIF is needed to protect against GVHD. Indeed, rLIF administration for a relative short period significantly protected mice from GVHD and prolonged mouse lifespan. In line with our findings in mice, an analysis of a publicly available dataset of transcriptome sequencing of rectosigmoid biopsies from GVHD patients (GSE134662)43 revealed a trend in a decrease (16/22 patients) in LIF levels in the colon of patients with refractory GVHD compared with LIF levels at the time of GVHD onset (supplemental Figure 11), suggesting that colon LIF levels are correlated with GVHD progression and has the potential to be developed as a biomarker for GVHD. However, the patient size in this dataset is relatively small, and future studies including more patients and normal control populations are needed for validation.
Damage to the GI tract is the main determinant of GVHD severity and lethality.5 Upon allo-BMT, donor T cells are recruited to the intestinal crypt region to attack ISCs.6 These findings highlight the importance of ISC protection for GVHD therapeutics. Results from this study demonstrated that rLIF promoted the ISC regeneration and proliferation after allo-BMT, suggesting that the effect of LIF on the ISC compartment contributes to the protective role of LIF in GVHD.
Allo–T cells infiltrate into recipient tissues after allo-BMT and get activated by APCs, which leads to inflammation and subsequent tissue damage and lethality in GVHD.5 A recent study reported that IECs act as APCs to induce donor T-cell activation and GVHD initiation.5 Here, we demonstrated that rLIF reduced the inflammation and donor T-cell infiltration in GVHD. IL-12 is a key inflammatory cytokine and inducer of GVHD.5,44 Our results showed that rLIF decreased IL-12–p40 induction by TBI in recipient DCs in MLNs, which then decreased MHC-II expression on IECs to inhibit Th1 differentiation and donor T-cell activation. rIL-12 administration largely abolished the protective effect of rLIF. Thus, our results revealed a previously unidentified role of LIF in regulating IL-12 expression, constituting a critical mechanism whereby LIF exerts its protective role in GVHD. The STAT family consists of 7 transcription factors.45 Previous studies, including ours, demonstrate STAT3 as a critical LIF downstream target in regulating many biological processes.46-49 LIF also promotes STAT4 activation to decrease intestinal inflammation in a mouse colitis model.50 Here, we found that LIF activated STAT1 in BMDCs to transcriptionally repress IL-12–p40. These results suggest a cell/tissue-type and context-specific role of LIF in regulating its downstream pathways to modulate different biological and pathological processes. STAT1 knockout mice can be used to validate the role of LIF in DCs in future studies.
Despite GVHD, allo–T cells remain the key to the success of BMT owing to their GVL activity. Of clinical importance, we evaluated the impact of LIF on donor T-cell–mediated GVL activity. Notably, LIF not only decreased GVHD, but also preserved GVL activity, supporting the potential of LIF as a therapeutic agent to protect against GVHD while preserving GVL. Although allogeneic donor Th1 cells were shown to induce both GVHD and GVL in mouse models, it was reported that Th1 blockade by targeting T-bet ameliorates GVHD without decreasing GVL activity, indicating the differential sensitivity of GVHD and GVL to Th1 reduction.51 Our results showed that LIF inhibited but did not completely block Th1 and T-cell activation, which could be the reason for LIF to protect against GVHD while preserving GVL.
Taken together, this study revealed a crucial role of LIF in protecting against GHVD by supporting ISC function and inhibiting donor T cell infiltration and activation via modulating the STAT1/IL-12/MHC-II axis. Our results suggest a potential translational application of LIF as a therapeutic agent to protect against GVHD while preserving GVL.
Acknowledgments
W.H. is supported by grants from the National Institutes of Health (NIH)/National Cancer Institute (NCI) (R01CA203965, R01CA260838), Department of Defense (W81XWH-18-10238), and the New Jersey Commission on Cancer Research (COCR22PRG004). Z.F. is supported by grants from the NIH/NCI (R01CA227912 and R01CA214746). P.X. is supported by a grant from the NIH (R21AI128264). This study was supported by the Flow Cytometry/Cell Sorting shared resource of Rutgers Cancer Institute of New Jersey (NIH/NCI P30CA072720).
Authorship
Contribution: J.W. carried out the experiments, analyzed data, and wrote the manuscript; C.-Y.C., X.Y., F.Z., J.L., and S.Z. carried out experiments; X.-Z.Y. assisted with experiments examining GVL; C.L. performed histological analyses; T.E.O. assisted with experiments analyzing IL-12 production; P.X. assisted with flow cytometric analysis of immune cell composition and activities; and Z.F. and W.H. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wenwei Hu, Rutgers Cancer Institute of New Jersey, Rutgers, The State University of New Jersey, 195 Little Albany St, New Brunswick, NJ 08903; e-mail: wh221@cinj.rutgers.edu; and Zhaohui Feng, Rutgers Cancer Institute of New Jersey, Rutgers, The State University of New Jersey, 195 Little Albany St, New Brunswick, NJ 08903; e-mail: fengzh@cinj.rutgers.edu.
References
Author notes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal