In this issue of Blood, Wang et al describe the beneficial effects of leukemia inhibitory factor (LIF) following allogeneic hematopoietic stem cell transplantation (alloHSCT) on both acute graft-versus-host disease (GVHD) affecting the gut and maintenance of graft-versus-leukemia (GVL) effects in mouse models.1 Administration of LIF before and/or after alloHSCT had multiple diverse effects. Protective effects on radiation protection/repair by intestinal stem cells (ISCs), induction of regulatory T cells (Tregs), suppression of class II expression on intestinal epithelial cells (IECs), and lower inflammatory/chemokine responses were all observed, thereby resulting in protection of the gut; however, GVL effects were maintained. These findings suggest a potentially new therapeutic for alloHSCT.
Perhaps no greater challenge in alloHSCT has existed than the ability to separate GVL from GVHD. This same paradigm also holds true for many cancer immunotherapies in which antitumor effects are sometimes foreshadowed by off-target toxicities, necessitating the need for immunosuppression at the expense of antitumor efficacy. The paradigm in alloHSCT is particularly complex because multiple pathways contribute to both GVHD and GVL processes, intimately linking the two.2 Both acute and chronic GVHD remain significant hurdles, limiting the efficacy of this procedure. Treatments continue to revolve around global immunosuppression, thereby dampening the inflammatory cascade and suppressing the extent of donor T-cell activation. There have been numerous attempts to modulate different GVHD pathways at each step: from altering cytoreductive conditioning procedures to minimize host tissue destruction and suppressing inflammatory responses targeting specific cytokines or pathways, to targeting donor T-cell responses or inducing specific immunosuppressive pathways and processes such that acute GVHD is mitigated but still allows for GVL effects.3 Although specific targeting of a particular pathway or cell type has been the prevailing emphasis, it is increasingly apparent, given the pleiotropic pathways involved in GVHD and GVL, that a multipronged attack would be more efficacious. This is where approaches involving LIF are attractive.
LIF is a member of the interleukin-6 (IL-6) family, originally described and named because of its effects on promoting differentiation of a murine leukemia cell line.4 Unfortunately, the name given to it has little to do with its now known dominant effects on embryonic stem cell biology and blastocyst implantation, as well as numerous functions on different tissues.4,5 Analogous to other similarly named cytokines, such as tumor necrosis factor, the name originally given to a factor does not always reflect its most dominant physiological functions. At times, the name can even be misleading, for example, LIF has been shown to promote progression of some tumors.4,6 LIF was also shown to have hematopoietic growth–promoting effects4 and has been previously evaluated as a myeloid growth factor after chemotherapy.7 Furthermore, LIF has been shown to exert significant immunological effects, specifically immune-suppressive effects mediated by Tregs, macrophage polarization effects, and overall anti-inflammatory properties.4,8 Along the same lines, due to its effects in stem cell repair and maintenance, Wang et al recently reported radioprotective effects of LIF in the gut as a prelude to this study.9 Given the dual properties of LIF on both tissue repair/radioprotection and immunosuppression, this would indicate potential usefulness in alloHSCT.
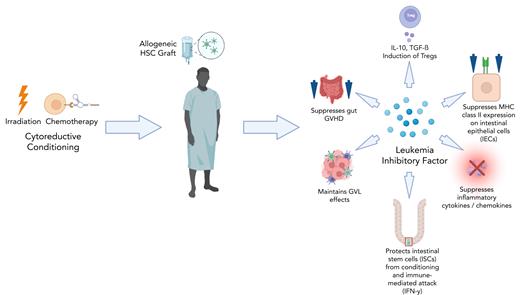
In this study, Wang et al assessed the role of LIF on GVHD and GVL processes using recipient LIF knockout mice (in which GVHD was increased) and then the effects of LIF administration on gut GVHD where protection was observed. They further demonstrated that ISCs were protected by LIF not only from irradiation but also from an interferon gamma–mediated attack using organoid models. LIF was also shown to suppress donor T-cell activation in part by blocking IL-12–mediated class II expression on IECs, which have been demonstrated to fuel the gut GVHD processes.10 Lower proinflammatory cytokine and chemokine induction was also observed. Finally, GVL activity was not only spared, but it also appeared that LIF alone may exert modest antileukemia effects. The study was attractive in that different mouse strain combinations, including haploidentical, were used, and multiple treatment regimens were attempted. As with most radioprotective regimens such as using IL-7 and keratinocyte growth factor, administration of LIF before conditioning resulted in the greatest GVHD protection. However, even delaying administration well after alloHSCT, still resulted in significant, albeit less, protection. The study therefore suggests that LIF can influence numerous pathways: protect stem cells, facilitate repair, suppress inflammation, modulate T-cell function, and possibly contribute to antileukemia effects (see figure).
Potential pathways by which LIF can protect from acute gut GVHD. IFN-γ, interferon gamma; IL-10, interleukin-10; MHC, major histocompatibility complex; TGF-β, transforming growth factor β.
Potential pathways by which LIF can protect from acute gut GVHD. IFN-γ, interferon gamma; IL-10, interleukin-10; MHC, major histocompatibility complex; TGF-β, transforming growth factor β.
What is needed to move this to the clinic? LIF has already been assessed clinically for other indications with a reasonable safety profile. Using LIF for alloHSCT and cancer is another matter though. In-depth preclinical studies are currently needed to address broader questions pertaining to alloHSCT. Are other acute GVHD target organs (liver, lung, skin) also directly protected or was it due to fewer gut GVHD effects? Are there windows for efficacy? Is chronic GVHD affected? Does it affect donor immune reconstitution? Does the cytoreductive conditioning protection observed with ISCs also affect malignant cells, and more importantly, is it dependent on the type of cancer? Antibodies to LIF are currently being examined for promoting anticancer immune responses, and ironically (given its name), LIF has been shown to promote cancer progression and metastasis in numerous tumors.6 It will therefore be critical to assess direct effects of LIF at various stages in hematologic malignancy progression of different human tumor types, as well as if the immunosuppressive properties of LIF can dampen GVL in some cancers that may not be as immunogenic as mouse tumor lines. Answers to all these questions along with further optimization of LIF administration are needed. Nonetheless, having a molecule exerting such diverse effects offers many opportunities, not only for alloHSCT but also in other instances in which either cytoreductive conditioning and/or immunotherapies are given (such as with chimeric antigen receptor–based approaches). We will just have to get around that unfortunate name and keep in mind the numerous and sometimes opposing effects different cytokines can have in GVHD and GVL processes and determine how best to exploit them. It may be that GVHD and GVL will always be tied together to some extent, but at least it may be possible to tip the balance in our patients’ favor.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal