Abstract
Anemia of inflammation (AI) is a highly prevalent comorbidity in patients affected by chronic inflammatory disorders, such as chronic kidney disease, inflammatory bowel disease, or cancer, that negatively affect disease outcome and quality of life. The pathophysiology of AI is multifactorial, with inflammatory hypoferremia and iron-restricted erythropoiesis playing a major role in the context of disease-specific factors. Here, we review the recent progress in our understanding of the molecular mechanisms contributing to iron dysregulation in AI, the impact of hypoferremia and anemia on the course of the underlying disease, and (novel) therapeutic strategies applied to treat AI.
Introduction
Anemia of inflammation (AI) is a highly prevalent comorbidity in patients with chronic inflammatory disorders, autoimmune diseases, infections, and cancer, which causes a severe additional burden on the recovery process from their primary disease. According to the latest report of the World Health Organization (WHO), anemia affects 24.8% of the worldwide population, of which most cases are caused by malnutrition.1 AI ranks in the second place, but its actual prevalence is difficult to assess because it often coexists with iron deficiency anemia (IDA). AI adversely affects disease outcomes and quality of life (QoL) through fatigue, dizziness, and functional cognitive impairment.2 The pathophysiology of AI is multifactorial and includes iron retention in macrophages of the reticuloendothelial system, reduction in circulatory half-life of erythrocytes, inadequate production and activity of erythropoietin (EPO), and impaired proliferation and differentiation of erythroid progenitor cells. The exact pathomechanisms involved often depend on the underlying primary disease. In this review, we focus on the prevalent diseases associated with AI and outline the most important molecular mechanisms that contribute to inflammatory hypoferremia and iron-restricted erythropoiesis.3,4
Diagnosis of AI
Iron-restricted erythropoiesis is an integral part of the pathophysiology of both AI and IDA, rendering differential diagnosis difficult specifically when both diseases coexist.5,6 Especially in low-income countries, where the burden of infectious diseases is high and true iron deficiency emerges because of poor nutritional iron availability and/or chronic blood loss, for example, owing to helminth infestations, AI and IDA frequently coaffect the same individuals. AI with combined IDA is also found in patients affected by inflammatory diseases and in those with chronic blood loss because of menstruation, repeated venesections, or disease-associated bleeding. As established by the WHO, anemia is defined as a hemoglobin (Hb) level of <120 g/L in women (<110 g/L in pregnant women) and <130 g/L in men. However, the dependency of the Hb value on multiple parameters, such as age, pregnancy, socioeconomic status, ethnicity, or altitude of living,7 complicates the universal definition of normal Hb concentration in healthy individuals.
The underlying causes of erythropoietic iron restriction differ between AI and IDA, and their knowledge is of importance for proper diagnosis and treatment. Iron restriction in AI mainly evolves from iron retention in macrophages and, only to a minor extent, from reduced dietary iron absorption. This state is referred to as functional iron deficiency and is hallmarked by iron storage in macrophages.3 It contrasts with absolute iron deficiency that occurs because of a disequilibrium between iron uptake, iron utilization, and iron loss that is observed in IDA.4 Notably, iron deficiency in response to EPO treatment (eg, when pulsatile erythropoiesis exceeds iron supply) or in adolescence may also cause “functional” iron deficiency, whereby, in this setting, tissue iron stores are depleted, as reflected by reduced circulating ferritin levels.8
Consistent with hypoferremia, individuals affected by AI show low transferrin saturation (TfSat) but normal or decreased transferrin levels. This differs from true IDA, in which transferrin levels are increased. Furthermore, ferritin levels are decreased in IDA, whereas ferritin levels are normal or increased owing to macrophage iron accumulation in AI.9 AI is further characterized by increased levels of inflammatory markers, such as interleukin 6 (IL-6) and C-reactive protein. Additional biochemical markers and hematologic indices are available for the differential diagnosis of AI and IDA and have been extensively reviewed elsewhere.10,11
Pathophysiological mechanisms of inflammation-induced hypoferremia
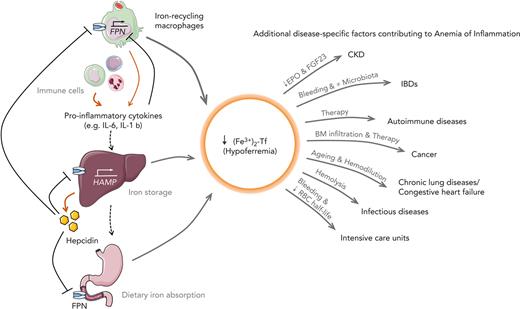
Inflammation induces functional iron deficiency, a state in which total body iron levels remain largely unchanged, and iron is redistributed into macrophages. Macrophages play a critical role in maintaining iron homeostasis by recycling 20 to 25 mg of iron from senescent red blood cells (RBCs) daily. Iron released from Hb during this process is either stored in the cellular iron storage protein ferritin or exported via ferroportin (FPN) into the bloodstream, where it is bound to transferrin. Iron-bound transferrin can be taken up by all tissues, including the erythropoietic bone marrow (BM). For comparison, only 1 to 2 mg of dietary iron is absorbed per day, which merely compensates for blood losses or the desquamation of skin or intestinal cells.12 Because iron export from macrophages and iron absorption from intestinal enterocytes are controlled by FPN, pathways reducing FPN expression affect body iron homeostasis and iron delivery to tissues. Inflammation induces the expression of the iron hormone hepcidin,13 which subsequently reduces FPN expression, and results in macrophage iron retention, reduced dietary iron absorption, and consequently the development of hypoferremia. The resulting restricted iron supply for erythropoiesis causes the gradual development of AI (summarized in Figure 1).
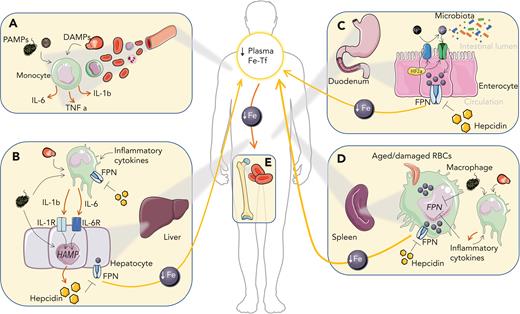
Iron-related mechanisms contributing to the generation of AI. (A) In circulation, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) activate myeloid cells leading to the production of proinflammatory cytokines. (B) Hepatocyte stimulation with proinflammatory cytokines, especially IL-6 and IL-1β, and PAMPs stimulates the expression of the liver iron hormone hepcidin, which promotes autocrine and paracrine iron retention due to the degradation of the iron exporter FPN. Kupffer cell stimulation with proinflammatory cytokines, PAMPs and DAMPs further feeds cytokine-mediated hepcidin upregulation in the liver. (C) Increased hepcidin levels in circulation block dietary iron import by degrading FPN. Local intestinal inflammation promotes HIF-2α (hypoxia-inducible factor 2α) stabilization and duodenal cytochrome b (DCYTB)/divalent iron transporter 1 (DMT1)–mediated iron absorption and may alter the composition of the intestinal microbiota. (D) In the spleen, where macrophages turn over large amounts of iron owing to the recycling of RBCs, iron accumulates in response to cytokines, hepcidin, PAMPs, and DAMPs, which decreases FPN-mediated iron export and affects additional pathways that cause iron retention. (E) Macrophage iron retention and decreased iron absorption reduce plasma iron levels and limit iron availability for erythroid progenitors, where iron is required for heme biosynthesis. As a consequence, anemia develops. Fe, iron; Fe-Tf, iron transferrin; TNF, tumor necrosis factor.
Iron-related mechanisms contributing to the generation of AI. (A) In circulation, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) activate myeloid cells leading to the production of proinflammatory cytokines. (B) Hepatocyte stimulation with proinflammatory cytokines, especially IL-6 and IL-1β, and PAMPs stimulates the expression of the liver iron hormone hepcidin, which promotes autocrine and paracrine iron retention due to the degradation of the iron exporter FPN. Kupffer cell stimulation with proinflammatory cytokines, PAMPs and DAMPs further feeds cytokine-mediated hepcidin upregulation in the liver. (C) Increased hepcidin levels in circulation block dietary iron import by degrading FPN. Local intestinal inflammation promotes HIF-2α (hypoxia-inducible factor 2α) stabilization and duodenal cytochrome b (DCYTB)/divalent iron transporter 1 (DMT1)–mediated iron absorption and may alter the composition of the intestinal microbiota. (D) In the spleen, where macrophages turn over large amounts of iron owing to the recycling of RBCs, iron accumulates in response to cytokines, hepcidin, PAMPs, and DAMPs, which decreases FPN-mediated iron export and affects additional pathways that cause iron retention. (E) Macrophage iron retention and decreased iron absorption reduce plasma iron levels and limit iron availability for erythroid progenitors, where iron is required for heme biosynthesis. As a consequence, anemia develops. Fe, iron; Fe-Tf, iron transferrin; TNF, tumor necrosis factor.
Inflammation-driven iron retention in macrophages is considered to represent an innate immune mechanism in vertebrates, also known as “nutritional immunity.” It limits iron availability for the proliferation and pathogenicity of extracellular microorganisms.14 However, some bacteria (eg, Salmonella typhimurium) circumvent the host’s defense strategy by invading and proliferating within phagosomal vacuoles of macrophages where they benefit from the increased intracellular iron content.15,16 On the other hand, increased hepcidin protects against siderophilic extracellular pathogens by limiting the appearance of non–transferrin-bound iron in the plasma that promotes their rapid growth.17,18 Accordingly, iron supplementation can promote the pathogenicity of such microbes. This problem is exemplified by iron supplementation studies in geographic areas where the burden of infectious diseases is high.19,20 For example, in the Pemba trial, routine dietary iron fortification in children from areas with high rates of malaria resulted in an increased risk of infection-driven morbidity and death,21 a result replicated in other populations.22-25 Despite this finding, the WHO recommends iron interventions in vulnerable populations, such as children and women of reproductive age,26-28 because benefits of iron interventions are generally considered to outweigh their risks.
Control of iron fluxes by FPN under inflammatory conditions
The only known iron exporter, FPN, is the gatekeeper of systemic iron fluxes. It is predominantly expressed in cell types with an iron export function, such as macrophages, duodenal enterocytes, and hepatocytes. During inflammation, cell surface expression of FPN is reduced by transcriptional and posttranslational mechanisms.
FPN is the target receptor for the liver-expressed iron hormone hepcidin, which upon binding occludes iron efflux29 and promotes internalization and degradation of FPN.30 Hepcidin transcription is activated by inflammatory cytokines that are secreted from innate immunity effector cells. IL-6–induced activation of the signal transducer and activator of transcription 3 (STAT3) pathway is a potent inflammation-driven pathway to induce hepcidin transcription,31,32 as shown both in humans and in animal models of AI.13 However, in complex inflammatory settings, Il6 or Hamp deficiency only partially protect against anemia triggered by injection of heat-killed Brucella abortus, supporting nonoverlapping roles of IL-6 and hepcidin in the etiology of AI.33 Similarly, neither IL-6 nor hepcidin were shown to be required for the development of anemia in aged mice.34 Thus, other cytokines and pathogen-driven immune stimuli contribute to hypoferremia and anemia development.35,36 For example, IL-1β contributes to hepcidin upregulation in cell-based assays and mice,37,38 although the responsible signaling mediators are still under discussion. Notably, IL-10 dose-dependently induced anemia in patients with inflammatory bowel disease (IBD) by promoting macrophage iron uptake and retention.39 Furthermore, PAMPs, such as bacterial cell wall components, have been proposed to directly promote hepcidin transcription upon receptor stimulation (eg, Toll-like receptors [TLRs]), in macrophages and hepatocytes, independent of the downstream-activated cytokines.40,41 Iron excess, in contrast, increases hepcidin levels via the bone morphogenetic protein/small mothers against decapentaplegic (BMP/SMAD) signaling pathway. Inflammatory activation of hepcidin transcription requires BMP/SMAD signaling because hepcidin upregulation does not occur in the absence of the BMP-responsive element in the hepcidin promoter.42-44 Furthermore, mice deficient for proteins mutated in hereditary hemochromatosis, such as hemojuvelin (a BMP receptor coreceptor) or HFE, show reduced BMP/SMAD signaling and attenuated hepcidin and hypoferremia responses in conditions of acute inflammation.45,46 Although most hepcidin is produced in hepatocytes, small amounts are also synthesized in monocytes and macrophages, where it acts in an autocrine manner to repress FPN-mediated iron export.47 Whether elevated hepcidin levels exert bactericidal activity in vivo is poorly understood. Its antimicrobial effect has been reported previously in vitro.48,49
Hepcidin promotes the degradation of FPN30 and occludes iron efflux by binding in a central cavity between the N and C domains.29 The affinity of hepcidin seems to be increased in the presence of iron, suggesting that iron-loaded FPN molecules are targeted for degradation. This notion is supported by observations that hepcidin-mediated, but not steady-state, FPN degradation is affected by cellular iron deficiency.50 Hepcidin binding induces lysine ubiquitination on an intracellular loop connecting the 2 halves of FPN.51 Specific ubiquitin ligases involved in FPN degradation have been described recently.52,53
A second prominent mechanism for inhibiting iron export via FPN and inducing hypoferremia is the direct transcriptional repression of Fpn by proinflammatory cytokines and bacterial components in macrophages and hepatocytes.54-57 Inflammation-mediated Fpn transcriptional downregulation alone can be suffice to cause hypoferremia54,57,58 and eventually AI in the absence of hepcidin activation.
Molecules released during tissue damage, named DAMPs or alarmins, arise in response to prototypical inflammatory immune responses and contribute to inflammation.59 These include intracellular components, such as metabolites or chromatin-associated proteins, that play physiological roles, but display “cytokine-like” functions in the extracellular space. DAMPs and alarmins can induce anemia, as exemplified by the finding that the binding of CpG-containing DNA to TLR9 in RBCs promotes erythrophagocytosis by splenic macrophages, resulting in acute anemia60 that may contribute to the reduced circulatory half-life of RBC found in AI. This is in line with the observation that activation of TLR7 and TLR9 signaling promotes the differentiation of specialized phagocytes responsible for increased erythrophagocytosis and the development of anemia.61
Disease entities frequently affected by AI
CKD
Chronic kidney disease (CKD) is a progressive, multifactorial disorder that can lead to renal failure over time. Anemia is highly prevalent in patients with CKD, ranging from 21% to 62% in different cohorts,62 and its prevalence increases upon CKD progression. Lower Hb levels correlate with a reduced QoL and the emergence of secondary complications, such as cardiovascular disease and mortality.63-65 In CKD, anemia caused by progressive kidney damage leads to reduced EPO production and hyporesponsiveness.63 In addition, uremic inhibitors of erythropoiesis (eg, indoxyl sulfate66), functional and/or absolute iron deficiency, and vitamin deficiencies (folate, cobalamine, vitamin D) contribute to anemia severity. Hepcidin levels in patients with CKD are elevated by different mechanisms. First, CKD results in reduced renal EPO secretion. EPO induces the hormone erythroferrone (ERFE) in erythroid progenitors, which enhances iron availability for erythropoiesis by suppressing hepcidin expression.67 Accordingly, in patients with CKD who are anemic, serum EPO and ERFE levels positively correlated. However, hepcidin levels vary between different cohorts,68-70 which is in line with the fact that in addition to regulation by ERFE, hepcidin levels are also affected by hypoxia and anemia-inducible factors, such as platelet-derived growth factor BB (PDGF-BB), growth-differentiation factor 15 (GDF15), or sexual hormones.71-74 Because hepcidin is cleared by the kidneys, the progression of renal disease is also associated with increased hepcidin,75,76 which is further aggravated by concomitant inflammation often linked to the underlying disease or dialysis.62,63 Additionally, blood loss during hemodialysis and repeated diagnostic blood sampling along with occult gastrointestinal bleeding contributes to iron losses that aggravate anemia and reduce EPO responsiveness.77 More recently, FGF23 (fibroblast growth factor 23) has been implicated in the establishment of AI in CKD. This bone-secreted hormone maintains phosphate and vitamin D homeostasis by targeting the kidney to modulate phosphate and calcium tubular reabsorption. Kidney damage decreases the expression of the renal FGF23 coreceptor αKlotho, thereby reducing the FGF23 response. To maintain mineral homeostasis, FGF23 levels increase.78,79 Elevated FGF23 levels, which also occur in response to iron deficiency and inflammation, affect hematopoietic stem cell differentiation and EPO regulation.80 In line with the proposed role of FGF23 in CKD, pharmacological inhibition of FGF23 stimulates erythropoiesis and counteracts anemia and iron deficiency in a CKD mouse model.81
In CKD, anemia is mainly treated with a combination of iron supplementation and erythropoiesis-stimulating agents (ESAs), a therapy that increases Hb levels and QoL.82 However, ∼20% of patients respond poorly, probably because of low-grade inflammation that directly affects hepcidin production and erythropoiesis.13,83
IBDs
IBDs, which mainly comprise ulcerative colitis and Crohn disease (CD), are conditions of cytokine-driven chronic gastrointestinal inflammation of multifactorial but distinct etiopathogenesis. Genetic susceptibility, environmental cues, and the gut microbiome can contribute to their pathogenesis. In CD, lesions mostly affect the terminal ileum, whereas in ulcerative colitis, they are limited to the colon and rectum.84 About two-thirds of patients with IBD present with anemia at diagnosis, which is considered the most common extraintestinal complication in IBD, significantly impairing patients’ QoL. The correction of anemia improves QoL independent of disease activity.85,86 Several mechanisms likely contribute to anemia in patients with IBD: (1) malnutrition (including iron) due to impaired intestinal absorption, (2) medication-associated toxicity (eg, azathioprine and methotrexate), (3) surgical procedures (eg, extensive bowel resection), (4) chronic intestinal bleeding from disease-associated ulcerations, and (5) inflammation.87
During the inflammatory process, infiltrating immune cells deplete oxygen in the local epithelial microenvironment, causing localized hypoxia.88 Hypoxia and proinflammatory cytokines promote HIF stabilization in phagocytes.89 Additionally, HIF activation increases iron absorption by controlling the messenger RNA expression of target genes responsible for duodenal iron transport (DMT1, Dcytb,90,91 and FPN92). Because iron export is inhibited by hepcidin-mediated FPN degradation, iron accumulates in enterocytes. Therefore, in IBDs, the sloughing of iron-loaded damaged enterocytes in response to inflammation and/or hypoxia affects the intestinal microbiome. Intestinal bacteria (eg, Lactobacilli) release metabolites that reduce duodenal iron absorption by suppressing intestinal HIF-2α activity to ensure sufficient bacterial iron supply.93 Intestinal iron accumulation in patients with active IBD94 affects gut microbiota diversity, their metabolic profile, and thus, disease activity.95,96 Accordingly, IV vs oral iron supplementation exerts contrasting effects on microbiome composition and disease activity in patients with IBD.95,97
Autoimmune diseases
Pathologic immune activation causes loss of tolerance and targeting of self-antigens. Manifestations range from asymptomatic disease to severe autoimmune disorders causing systemic and local inflammation that affect multiple organs. Both genetic and environmental factors contribute to autoimmunity. Although the exact disease mechanisms differ between various autoimmune disorders, increased levels of inflammatory mediators (eg, TNF family members and interleukins) or JAK/STAT pathway activation are commonly observed.98,99
AI has been reported in 30% to 60% of patients with rheumatoid arthritis (RA), whereas IDA only occurs in a minority of patients, mainly because of therapy-induced, gastrointestinal, or urogenital blood losses. Disease severity is associated with reduced Hb levels and a higher prevalence of anemia.100,101 Both autoimmune disease per se or side effects of immunosuppressive therapies can negatively affect BM cellularity, erythropoiesis, and erythrocyte half-life.99,102 IL-6 plays a central role in RA pathogenesis103 because it promotes joint inflammation and the development of anemia via hepcidin induction. Consistently, patients with RA show elevated hepcidin levels that correlate with disease activity, as well as with comorbidities such as coronary atherosclerosis and osteoporosis.104-106 The most effective way to combat AI in autoimmune diseases is to treat the underlying inflammation with (1) systemic immunosuppressive drugs, such as corticosteroids or methotrexate; or (2) cytokine or immune-signaling neutralizing antibodies (eg, TNFα, IL-6, IL-17, IL-23), JAK inhibitors, or combinations thereof.103,107,108 However, in a substantial proportion of patients, these therapeutic strategies fail, and both inflammation and anemia persist. These patients may receive IV iron sometimes in combination with ESAs; however, the efficacy of these therapies is limited in more advanced inflammatory disease states.102,109
Cancer
Anemia is observed in 40% to 80% of patients with cancer,110,111 varying in severity depending on tumor site, stage, patient age, and treatments.112 It is one of the most frequent secondary problems and is closely related to disease progression. Anemia of cancer is caused by multiple mechanisms, including inflammation, BM invasion by tumor cells, and vitamin deficiencies. It is aggravated by anticancer therapies, which often damage erythroid progenitor cells and/or reduce erythrocyte half-life.113,114 Moreover, tumor cells can produce cytokines that negatively affect erythroid progenitor cell differentiation. Patients with cancer often present with increased hepcidin levels, and accordingly, hepcidin neutralization with monoclonal antibodies promotes iron mobilization.115
Despite high hepcidin levels116 and functional iron deficiency, several clinical trials demonstrated that IV (but not oral) iron administration increases the hematopoietic response.117 Iron chelation has been tested in other studies to restrict the growth of very iron-avid tumors,118 despite possible worsening of patients’ anemia. Iron homeostasis must be balanced in patients with cancer to ensure iron supply for erythropoiesis without promoting tumor growth. To date, end point data on the effects of iron supplementation or iron chelation are missing to fully estimate treatment effects on the course of the malignant disease and concurrent anemia.
Chronic lung diseases
Chronic lung diseases affect respiratory capacity, cardiovascular functionality, and thus, blood oxygenation with the emergence of tissue hypoxia over time. Patients with chronic lung diseases, such as cystic fibrosis, pulmonary arterial hypertension, or chronic obstructive pulmonary disease (COPD), are often anemic, with or without iron deficiency, and iron deficiency and/or anemia can aggravate the pulmonary disease.119-124 Although the prevalence may vary across different disease entities and populations, the negative impact of anemia on QoL is almost universal. Anemia is best studied in COPD, a disease hallmarked by persistent airflow limitation due to chronic inflammation, oxidative stress, and an imbalance of proteases and antiproteases.125 The prevalence of anemia in patients with COPD varies between 7.5% and 33% and increases over time with worsening functional performance and increased symptoms.126-128 WHO Hb cutoffs may fail to accurately determine the prevalence of anemia in these patients because of the underlying hypoxemia. Hypoxia increases EPO levels and erythropoiesis and represses hepcidin by ERFE-dependent mechanisms to increase iron availability for erythroid precursors.129 Moreover, hypoxia and iron deficiency affect pulmonary remodeling and increase pulmonary pressures with negative effects on cardiopulmonary function.122 Importantly, secondary polycythemia resulting from persisting hypoxia and/or inhalative noxes may further affect pulmonary circulation and oxygenation.130,131
Systemic inflammation can be causative of COPD, with the pulmonary manifestation being part of multiple organ involvement, or a consequence of the disease due to systemic “spill-over” of pulmonary inflammation and infections, especially during exacerbations.132,133 Increased IL-6 and hepcidin levels in patients with COPD are likely the most relevant iron-related mechanisms that lead to the establishment of anemia that is often combined with absolute iron deficiency.128,134,135 Additionally, age contributes to anemia in patients with COPD,126 and the prevalence of anemia is higher in older adults.136 Because hepcidin levels do not increase with age, this may reflect a preponderant contribution of other mechanisms to the establishment of AI, such as clonal hematopoiesis of indeterminate potential and “inflammaging,” but also related to weaning renal and liver function.137 The intricate relationship between aging, inflammation, and anemia has recently been reviewed by Girelli et al.137,138
Despite the high prevalence of anemia in patients with COPD, it is frequently disregarded as a treatable problem in clinical practice. In 1 trial, treatment of anemia with a combination of IV iron and ESAs improved dyspnea and overall QoL.119 However, the long-term effects of such interventions on cardiovascular activity or pulmonary circulatory pressures are lacking. Importantly, it is essential to provide a good diagnostic workup of the causes of anemia in COPD because treatment strategies and efficacy may differ according to the presence of absolute or functional iron deficiency or other coexisting pathologies affecting erythropoiesis.139 Accordingly, treatment failure in a subgroup of patients with COPD may be explained by EPO resistance or iron trapping in macrophages as a consequence of inflammation.134,140
Congestive heart failure (CHF)
CHF is a chronic progressive condition in which the heart cannot pump enough blood to meet the body’s requirements. Anemia and iron deficiency frequently affect patients with CHF (∼30% in stable and ∼50% in hospitalized patients), and its prevalence is positively associated with the progression of the disease.141 The major cause of anemia in CHF is AI, but combined true iron deficiency and hemodilution along with reduced renal function are also of importance. Specifically, patients with CHF and reduced ejection fraction have increased markers of inflammation,142 and accordingly, increased hepcidin levels are observed in patients with more advanced disease.143 Importantly, IV iron supplementation can improve clinical parameters and the prognosis of CHF.144 Regular evaluation of iron status and treatment of eventual iron deficiency is recommended by current guidelines.145
Infectious diseases
In high-income countries, infectious diseases are less commonly considered as major contributors to the prevalence of AI,146 although AI is frequently found in association with acute or chronic infections.147 With the emergence of COVID-19, the interplay between infection, iron homeostasis, and anemia received increased attention. Meta-analyses reported a 25% prevalence of anemia in patients with COVID-19,148 whereby 70% of patients who were anemic had AI.149 In hospitalized patients, the presence/development of anemia and/or functional iron deficiency was associated with more severe disease and negative outcome of COVID-19.150-152 As in other infectious diseases, severe COVID-19 resulted in a hyperinflammatory state with high serum ferritin levels and reduced TfSat.149,153 In a study performed in hospitalized patients with COVID-19, IL-6 significantly correlated with hepcidin levels. However, it was only poorly correlated with serum iron levels, suggesting that additional hepcidin-independent mechanisms contribute to the establishment of hypoferremia and AI.154
HIV infections, which cause AIDS, are a global health care challenge. Anemia is highly prevalent (47% in adults155) and one of the most common hematological anomalies in patients with AIDS, affecting clinical progression and QoL.156,157 Anemia is far more frequent in patients not receiving antiretroviral therapy but may also be found in patients undergoing treatment owing to therapy-related toxicity or nutritional deficiencies.156,158 In patients with advanced AIDS, hepcidin levels directly correlate with AI prevalence and inversely with CD4 T-cell counts. The increased hepcidin levels in progressed HIV infections159 result in iron retention in infected cells, which may further enforce viral replication.160 Control of infection, improvement of inflammation, and correction of iron imbalances upon antiretroviral therapy cannot always resolve anemia,161 indicating a multifactorial etiology.
Intensive care units (ICUs)
Anemia is common in critically ill patients admitted to the ICU. Two-thirds of the patients present with only mild anemia upon admission, whereas after 1 week, up to 97% of the patients are anemic.162,163 Although ICU anemia is associated with worse outcomes, it is still controversial whether this reflects a causal relationship or if anemia is just an indicator of disease severity.164,165 The etiopathogenesis of anemia in ICU-admitted patients is multifactorial and involves bleeding-associated and iatrogenic loss of RBCs, along with the typical features of AI and decreased RBC circulatory half-life. The latter may be linked to bacterial DNA sensing by TLR9 on RBCs, subsequently promoting erythrophagocytosis.60 Patients from multiple cohorts show decreased plasma iron, transferrin, and Hb levels, as well as increased levels of ferritin, IL-6, and hepcidin, and these parameters were linked to the outcome from sepsis.163,166,167 Furthermore, a high TfSat in these patients is associated with reduced survival, possibly reflecting either a pathogenic effect of higher iron availability or inappropriate macrophage iron handling after transfusion.167,168 Recent results also suggest that during resolution of sepsis and progression to chronic critical illness, persistent inflammation is triggered by continuous exposure to alarmins and danger-associated molecular patterns that skew hematopoiesis toward myelopoiesis and chronic anemia.169,170
Guidelines emphasize the need for limited blood losses and recommend conservative treatment of anemia with RBC transfusions with low thresholds for the initiation of therapy, depending also on the underlying comorbidities (Hb, 7-8 g/dL).171,172 This is in line with the observation that restrictive RBC transfusion strategies were associated with lower mortality than liberal blood transfusion strategy. Notably, a randomized controlled trial of IV iron did not show benefit regarding anemia treatment in ICU patients (IRONMAN study),173 which may be linked to inflammation-driven iron trapping in macrophages. This is in agreement with a meta-analysis indicating that iron supplementation in ICU patients did not improve RBC requirements.174 The addition of ESAs has been shown to result in reduced RBC needs in some, but not all, studies.175 However, in 1 study, reduced mortality with ESA treatment was observed, which may be linked to anti-inflammatory activities of EPO in critically ill patients.176,177 Interestingly, in a study involving 399 patients with prolonged ICU admission and the need for anemia treatment, a hepcidin and EPO level–guided diagnostic protocol demonstrated reduced mortality at 3 months in treated patients.178 Thus, iron supplementation and/or ESA may be beneficial in subgroups of critically ill patients and in those with prolonged ICU stay. However, more powerful trials are required to clearly identify the patients who will benefit from the abovementioned strategies.
Current and future treatment options in AI
The most effective treatment for AI is the cure for the underlying disease and the resolution of inflammation. In those cases where this is not possible, particularly when anemia is mild and does not severely affect the patient’s QoL, treatment may be omitted. Unfortunately, data are lacking on how concomitant anemia and/or iron deficiency affect the course or the symptoms of the underlying disease. In principle, 3 treatment options exist for AI: RBC transfusion, iron supplementation, and ESAs (extensively discussed in recent reviews).3,4,139,179 RBC transfusions are restricted to patients with severe anemia (Hb, <8 g/L) and are only a temporary strategy because of the increased mortality in specific conditions.180,181 ESAs, such as erythropoietin, stimulate the BM to produce more RBCs. These drugs counteract the antiproliferative effects of proinflammatory cytokines and stimulate iron uptake and heme biosynthesis in erythroid precursors. However, ESAs were associated with unfavorable clinical outcomes, particularly in patients with an initially poor response.182 Oral and IV iron supplementation is used to combat erythropoietic iron restriction. However, iron absorption and export from macrophages require the surface expression of FPN, which is inhibited by inflammation. Thus, the efficacy of these treatments is often not satisfactory, especially in patients with more advanced inflammation and functional iron deficiency, given the ensuing substantial side effects.109 To overcome the disadvantages of conventional treatments in AI, novel strategies are under development that directly benefit from the growing knowledge of the pathophysiology underlying AI (Table 1).
Drugs in trials for the treatment of AI
| Disease context . | Drug . | Type of drug . | Target . | Beneficial effects on AI . | Stage . |
|---|---|---|---|---|---|
| CKD | P2D7KK | Antibody | IL-1β | ↓ inflammation ↑ renal function ↓ anemia | Preclinical183 |
| KY1070 | Antibody | BMP6 | ↓ hepcidin ↑ erythroid response | Preclinical184 | |
| Ziltivekimab | Antibody | IL-6 | ↓ inflammation ↓ ESA requirements ↓ iron restriction | Clinical (phase 1/2)185 | |
| PRS-080#22 | Anticalin protein | Hepcidin | ↓ hepcidin ↑ iron mobilization | Clinical (phase 1)186 | |
| LY2928057 | Antibody | FPN/hepcidin binding | ↑ serum iron ↓ Hb decline after discontinuation of standard treatment | Clinical (phase 1/2)187 | |
| Vadadustat | Small molecule | HIF-PH | Noninferior to ESAs in the maintenance of Hb concentrations | Clinical (phase 3)188 | |
| Roxadustat | Small molecule | HIF-PH | Noninferior to ESAs in patients undergoing dialysis | Clinical (phase 3)189 | |
| Daprodustat | Small molecule | HIF-PH | Noninferior to ESAs for increase in Hb | Clinical (phase 3)190 | |
| CD | Infliximab | Antibody | TNFα | ↓ hepcidin ↑ serum iron ↑ Hb | FDA/EMA approved191-193 |
| RA | Tocilizumab | Antibody | IL-6 receptor | ↓ inflammation ↓ hepcidin ↑ Hb, RBC counts | FDA/EMA approved194,195 |
| Advanced cancer | Lactoferrin | Iron-binding protein | Similar efficacy for oral lactoferrin and for IV iron, combined with rHuEPO | Clinical (phase 1)196 | |
| ALD518 | Antibody | IL-6 | ↑ Hb | Clinical (phase 2)197 | |
| Endotoxemia | Lexaptepid | Small molecule | Hepcidin | Transient ↑ serum iron | Clinical (phase 1)198 |
| Disease context . | Drug . | Type of drug . | Target . | Beneficial effects on AI . | Stage . |
|---|---|---|---|---|---|
| CKD | P2D7KK | Antibody | IL-1β | ↓ inflammation ↑ renal function ↓ anemia | Preclinical183 |
| KY1070 | Antibody | BMP6 | ↓ hepcidin ↑ erythroid response | Preclinical184 | |
| Ziltivekimab | Antibody | IL-6 | ↓ inflammation ↓ ESA requirements ↓ iron restriction | Clinical (phase 1/2)185 | |
| PRS-080#22 | Anticalin protein | Hepcidin | ↓ hepcidin ↑ iron mobilization | Clinical (phase 1)186 | |
| LY2928057 | Antibody | FPN/hepcidin binding | ↑ serum iron ↓ Hb decline after discontinuation of standard treatment | Clinical (phase 1/2)187 | |
| Vadadustat | Small molecule | HIF-PH | Noninferior to ESAs in the maintenance of Hb concentrations | Clinical (phase 3)188 | |
| Roxadustat | Small molecule | HIF-PH | Noninferior to ESAs in patients undergoing dialysis | Clinical (phase 3)189 | |
| Daprodustat | Small molecule | HIF-PH | Noninferior to ESAs for increase in Hb | Clinical (phase 3)190 | |
| CD | Infliximab | Antibody | TNFα | ↓ hepcidin ↑ serum iron ↑ Hb | FDA/EMA approved191-193 |
| RA | Tocilizumab | Antibody | IL-6 receptor | ↓ inflammation ↓ hepcidin ↑ Hb, RBC counts | FDA/EMA approved194,195 |
| Advanced cancer | Lactoferrin | Iron-binding protein | Similar efficacy for oral lactoferrin and for IV iron, combined with rHuEPO | Clinical (phase 1)196 | |
| ALD518 | Antibody | IL-6 | ↑ Hb | Clinical (phase 2)197 | |
| Endotoxemia | Lexaptepid | Small molecule | Hepcidin | Transient ↑ serum iron | Clinical (phase 1)198 |
Despite the efficacy of hypoxia-inducible factor prolyl hydroxylases inhibitors (HIF-PHIs) to ameliorate anemia in the context of CKD, these drugs have not been licensed for clinical use in the United States (FDA), whereas they were approved by the European authorities (EMA). The FDA justified its decision by the fact that HIF-PHIs activate numerous HIF target genes in different organs. Specifically, the higher frequency of thromboembolic events, acceleration of diabetic retinopathy, or progression of malignancy in some patients treated with HIF-PHIs raised concerns.199,200
CD, Crohn disease; EMA, European Medicines Agency; FDA, Food and Drug Administration; HIF-PH, hypoxia-inducible factor prolyl hydroxylase; rHuEPO, recombinant human erythropoietin.
Outlook
Despite our dynamically expanding understanding of the molecular mechanisms underlying AI, the impact of anemia and/or iron deficiency on the course of the underlying disease and the effect of therapeutic strategies remain unclear. Suitable diagnostic algorithms for the differentiation of AI with or without concomitant IDA must be developed because these clinical states require different therapeutic approaches. Although RBC transfusions are indicated in emergency situations of life-threatening severe anemia, iron supplementation and ESA therapy remain the mainstay of therapy. However, in the setting of ongoing inflammation, and also due to disease-specific factors, their therapeutic effect can remain unsatisfactory.
Therefore, a personalized approach based on knowledge of the underlying pathophysiological mechanisms, clinical features of the underlying disease, and diagnostic results is essential to determine the optimal therapeutic approach (summary in Figure 2). Recent scientific discoveries have paved the way for novel emerging therapies, including those targeting the hepcidin/FPN axis, hypoxia-induced pathways, or immune cell signaling cascades. We are urgently awaiting their clinical evaluation. These therapies must be evaluated for their capacity to overcome AI-driven macrophage iron retention and/or immune-mediated repression of erythropoiesis and how such effects will affect the course of the specific disease underlying AI.
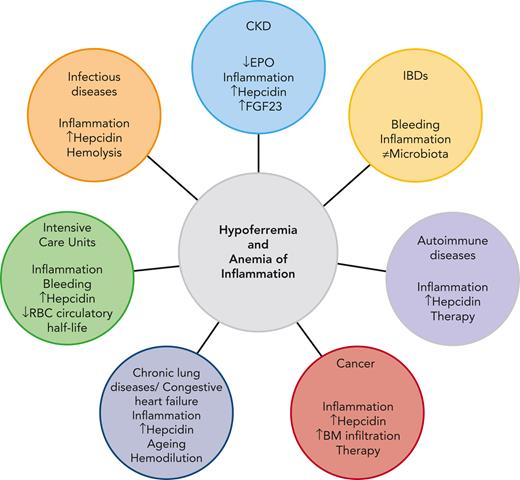
Leading pathophysiological mechanisms contributing to hypoferremia and AI in CKD, IBDs, autoimmune diseases, cancer, chronic lung diseases, CHF, infectious diseases,and ICUs.
Leading pathophysiological mechanisms contributing to hypoferremia and AI in CKD, IBDs, autoimmune diseases, cancer, chronic lung diseases, CHF, infectious diseases,and ICUs.
Acknowledgments
The authors thank Matthias W. Hentze for the critical reading of the manuscript.
M.U.M. acknowledges funding from the Deutsche Forschungsgemeinschaft (SFB1118; FerrOs-FOR5146) and the Federal Ministry of Education and Research (NephrESA Nr 031L0191C; DZL TLRC-H). O.M. received support from the European Hematology Association (RG66) and by an Olympia Morata-Programme Fellowship given by the Medical Faculty of the University of Heidelberg. G.W. was supported by the Christian Doppler Society.
Authorship
Contribution: O.M. and M.U.M. were responsible for conception and design; and O.M., G.W., and M.U.M. wrote the manuscript and approved its final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martina U. Muckenthaler, Im Neuenheimer Feld 350, 69120 Heidelberg, Germany; e-mail: martina.muckenthaler@med.uni-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal