Key Points
After a median of 10.0 years of follow-up, low-count MBL had a significant 4.3-fold increased risk of lymphoid malignancies.
After a median of 34.4 months of follow-up, there was no evidence of an association of low-count MBL with overall survival.
Abstract
Monoclonal B-cell lymphocytosis (MBL) is a common hematological premalignant condition that is understudied in screening cohorts. MBL can be classified into low-count (LC) and high-count (HC) types based on the size of the B-cell clone. Using the Mayo Clinic Biobank, we screened for MBL and evaluated its association with future hematologic malignancy and overall survival (OS). We had a two-stage study design including discovery and validation cohorts. We screened for MBL using an eight-color flow-cytometry assay. Medical records were abstracted for hematological cancers and death. We used Cox regression to evaluate associations and estimate hazard ratios and 95% confidence intervals (CIs), adjusting for age and sex. We identified 1712 (17%) individuals with MBL (95% LC-MBL), and the median follow-up time for OS was 34.4 months with 621 individuals who died. We did not observe an association with OS among individuals with LC-MBL (P = .78) but did among HC-MBL (hazard ratio, 1.8; 95% CI, 1.1-3.1; P = .03). Among the discovery cohort with a median of 10.0 years follow-up, 31 individuals developed hematological cancers with two-thirds being lymphoid malignancies. MBL was associated with 3.6-fold risk of hematological cancer compared to controls (95% CI, 1.7-7.7; P < .001) and 7.7-fold increased risk for lymphoid malignancies (95% CI:3.1-19.2; P < .001). LC-MBL was associated with 4.3-fold risk of lymphoid malignancies (95% CI, 1.4-12.7; P = .009); HC-MBL had a 74-fold increased risk (95% CI, 22-246; P < .001). In this large screening cohort, we observed similar survival among individuals with and without LC-MBL, yet individuals with LC-MBL have a fourfold increased risk of lymphoid malignancies. Accumulating evidence indicates that there are clinical consequences to LC-MBL, a condition that affects 8 to 10 million adults in the United States.
Introduction
Monoclonal B-cell lymphocytosis (MBL) is a premalignant condition to chronic lymphocytic leukemia (CLL). It has a prevalence of 3% to 12% in White individuals from the general population but increases to 22% in relatives of CLL patients.1-4 MBL is characterized by a circulating population of clonal B cells with an absolute clonal B-cell count <5 × 109/L and no evidence of lymphadenopathy, organomegaly, or cytopenias.4,5 MBL can be classified by the immunophenotype (CLL-like MBL, non–CLL-like MBL, and atypical MBL) and by the size of the clone: low-count MBL (LC-MBL; clonal B-cell count <0.5 × 109/L) or high-count MBL (HC-MBL; clonal B-cell count between 0.5 and 5 × 109/L).4,6,7
A number of studies have shown the clinical outcomes among individuals with HC-MBL who were identified in clinical practice, including an increased risk of progression to CLL requiring therapy,8-10 increased risk of solid tumors,11 reduced humoral immune response to vaccinations,12 and increased risk of hospitalizations due to infections.13 Few studies have evaluated the clinical outcomes among individuals identified to have MBL (LC- or HC-MBL) through screening. In an MBL screening study of 448 individuals from families with multiple members with CLL, we reported that LC-MBL preceded CLL diagnosis by more than 8 years, and individuals with LC-MBL progressed to CLL at a rate of 1.1%/year.1 We also showed in a separate screening cohort of 1045 individuals from the Mayo Clinic Biobank that individuals with LC-MBL have a 1.6-fold increased risk of hospitalizations due to infections compared to individuals without MBL.14 A small screening study from Spain including 65 individuals with LC-MBL and 290 individuals without MBL reported a significantly shorter overall survival (OS) among those with MBLs.15 Herein, we evaluated the natural history of MBL and its association with future hematological cancers and OS in a large MBL screening cohort of 10 139 individuals from the Mayo Clinic Biobank.
Methods
Study cohort
This study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center, and participants provided written informed consent. Study participants were identified from the Mayo Clinic Biobank, a large-scale biorepository of 56 959 adult patients recruited from primary care–based clinics between 2009 to 2016.16,17 All participants filled out a self-reported health history questionnaire and provided blood specimens. In a random sample of 10% of participants, a sodium heparin tube was collected and processed by Ficoll-Hypaque separation of the peripheral blood mononuclear cells followed by a slow freeze processing, allowing us the ability to screen for MBL. Of these, we excluded individuals with a prior medical record of or a self-reported history of hematologic cancer and those younger than 40 years of age, due to low incidence of MBL, resulting in 4207 individuals in our MBL discovery cohort (supplemental Figure 1, available on the Blood website). Using this discovery cohort, we previously reported the association of MBL with various self-reported factors (individuals were not aware of their MBL status at the time they provided this information) including lifestyle factors, personal medical history, occupational history, and family history of leukemia or lymphoma; we also evaluated associations with inherited common polymorphisms.18
Starting in 2017, we assembled an MBL validation cohort from the individuals participating in the Mayo Clinic Biobank who were not randomized to have slow-frozen peripheral blood mononuclear cells, resided in the counties surrounding Mayo Clinic, and were alive and had no prior history of hematologic malignancy based on medical record review and self-reported questionnaire. As of March 2022, a total of 12 975 individuals were invited to participate in our validation cohort and 8231 (63%) consented. Of the consented individuals, 5932 provided a blood sample and had MBL screening performed as time of this report (supplemental Figure 1).
Finally, to evaluate the incidence and natural history of MBL, we invited all individuals from the discovery cohort who were alive as of 2017 and resided in the surrounding 27 counties of Mayo Clinic to provide a follow-up blood sample. A total of 1471 individuals were invited into this follow-up study and 745 (51%) consented; of these, 626 patients provided a blood sample that has been screened (supplemental Figure 1).
MBL screening
To screen for MBL, we used a sensitive eight-color (CD38, CD45, Kappa, Lambda, CD19, CD23, CD5, and CD20) flow cytometry assay capable of detecting clonal B-cell event to the 0.005% level with a minimum of 20 mononuclear cells required.14 All cases of MBL were confirmed by a Mayo Clinic hematopathologist who specializes in lymphoid malignancy. This pathologist and the technologist performing the MBL screening assay were blinded to all clinical outcomes of the study participants. We defined 3 MBL immunophenotypes (1) CLL-like MBL (CD5+, CD20dim), (2) atypical MBL (CD5+, CD20+), and (3) non–CLL-like MBL (CD5−, CD20+). Blood counts were ascertained from medical records within 1 year of sample used for MBL screening. Because not all biobank participants had a complete blood count obtained within 1 year of research samples, we classified individuals by the size of the clone relative to total B cells, with HC-MBL defined as those individuals who had a percent clonal B-cell count ≥85% of the total B-cell count as we have done previously.1,19
Statistical analysis
Our end points were progression to hematological cancer and OS. For OS, all individuals were followed from sample date to the earliest of death, loss to follow-up, withdrawal from the study, or 1 March 2022. To identify incident hematological cancers, we used the Mayo Clinic CLL Resource and the Mayo Clinic Lymphoma Specialized Program of Research Excellence Molecular Epidemiological Resource, both of which ascertain individuals seen at Mayo Clinic with any clonal B-cell leukemic population and lymphoma, respectively. For individuals not seen at Mayo Clinic, we used the Rochester Epidemiology Project (REP) to identify hematological cancers through medical record abstraction using the International Classification of Diseases codes. The REP is a population-based medical records linkage system with access to the complete (inpatient and outpatient) medical records from all medical facilities in the 27-county area surrounding Mayo Clinic.20 Because of the length of follow-up, only individuals from the discovery cohort who were residents of the 27-county area surrounding Mayo Clinic at the time of sample collection were included in the analyses with progression to hematological cancer (N = 1651), and they were followed from sample date to the earliest of progression to hematological cancers, death, loss to follow-up, withdrawal, or 31 December 2021 (the last date REP has follow-up information). Cumulative incidence of hematological cancer adjusting for the competing risk of death was calculated. Analyses of individuals with MBL and controls (individuals screened negative for MBL) were assessed for OS by adjusting for age and sex using inverse weights methods. Plots display Wald-adjusted P-values. Cox regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), adjusted for age and sex. The Cochran-Armitage trend test was used to determine if the proportion of MBL cases in each age group followed a linear trend. Comparisons of demographic factors between MBL and controls were performed using χ2 and rank sum tests. All statistical tests were two-sided and P < .05 was considered significant. Analyses were performed with SAS software (version 9.4, SAS Institute) and figures were created using R 3.6.2 (R Foundation for Statistical Computing).
Results
Across the discovery and validation cohorts, we screened 10 139 individuals 40 years of age or older (median age, 66 years; 39% male) for MBL. We identified prevalent MBL in 1712 (17%), 8174 (81%) individuals were negative for MBL (ie, controls), and 253 (2%) individuals had insufficient cells for flow cytometry. Because MBL prevalence was similar across discovery (15%) and validation cohorts (19%), results were combined across cohorts. Demographic characteristics and blood counts are summarized in Table 1. The median age of individuals with MBL was 72 years compared to 65 years in controls (P < .001). The prevalence of MBL increased with age ranging from 4% among those between 40 and 49 years of age to 42% among those 90+ years of age (P < .001) (Figure 1A). The prevalence of MBL differed between males (22%) and females (14%) (P < .001) (Figure 1B). Variability by self-reported race (Figure 1C) was observed but not statistically significant (P = .07). The majority (95%) of the MBLs identified was considered LC-MBL; 93 individuals were considered HC-MBL.
Characteristics of study participants
| . | MBL . | Control . | Total . |
|---|---|---|---|
| N | 1712 | 8174 | 9866 |
| Median age at sample (range), y | 72 (41-98) | 65 (40-101) | 66 (40-101) |
| Male | 865 (50.5) | 2981 (36.1) | 3846 (38.9) |
| Race | |||
| White/non-Hispanic | 1630 (95.3) | 7772 (95.1) | 9402 (95.1) |
| Asian | 11 (0.6) | 72 (0.9) | 83 (0.8) |
| African American | 1 (0.1) | 35 (0.4) | 36 (0.4) |
| Other | 69 (4.0) | 295 (3.6) | 364 (3.7) |
| High count MBL | 93 (5.4) | — | — |
| >1 MBL immunophenotype | 85 (5.0) | — | — |
| Median hemoglobin (range), g/dL | N = 1249 | N = 5554 | |
| 13.9 (7.3-17.9) | 13.7 (4.8-19.3) | ||
| Median platelet count (range), ×109/L | N = 1248 | N = 5547 | |
| 220 (23-512) | 231 (45-871) | ||
| Median white blood count (range), ×109/L | N = 1247 | N = 5547 | |
| 6.4 (2.3-28.2) | 6.2 (1.8-22.5) | ||
| Median absolute lymphocyte count (range), ×109/L | N = 1126 | N = 4923 | |
| 1.6 (0.2-11.1) | 1.6 (0.1-7.9) |
| . | MBL . | Control . | Total . |
|---|---|---|---|
| N | 1712 | 8174 | 9866 |
| Median age at sample (range), y | 72 (41-98) | 65 (40-101) | 66 (40-101) |
| Male | 865 (50.5) | 2981 (36.1) | 3846 (38.9) |
| Race | |||
| White/non-Hispanic | 1630 (95.3) | 7772 (95.1) | 9402 (95.1) |
| Asian | 11 (0.6) | 72 (0.9) | 83 (0.8) |
| African American | 1 (0.1) | 35 (0.4) | 36 (0.4) |
| Other | 69 (4.0) | 295 (3.6) | 364 (3.7) |
| High count MBL | 93 (5.4) | — | — |
| >1 MBL immunophenotype | 85 (5.0) | — | — |
| Median hemoglobin (range), g/dL | N = 1249 | N = 5554 | |
| 13.9 (7.3-17.9) | 13.7 (4.8-19.3) | ||
| Median platelet count (range), ×109/L | N = 1248 | N = 5547 | |
| 220 (23-512) | 231 (45-871) | ||
| Median white blood count (range), ×109/L | N = 1247 | N = 5547 | |
| 6.4 (2.3-28.2) | 6.2 (1.8-22.5) | ||
| Median absolute lymphocyte count (range), ×109/L | N = 1126 | N = 4923 | |
| 1.6 (0.2-11.1) | 1.6 (0.1-7.9) |
Values are n (%) unless otherwise stated.
Prevalence of MBL by age groups (A), by sex (B), and by self-reported race (C).
Prevalence of MBL by age groups (A), by sex (B), and by self-reported race (C).
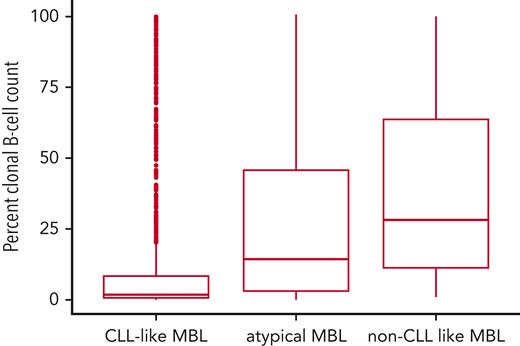
We identified 85 MBL individuals with more than 1 MBL immunophenotype. The most common immunophenotype was CLL-like MBL (n = 1451 individuals), followed by non–CLL-like (n = 257 individuals), and then atypical MBL (n = 92 individuals). The distribution of the percent clonal B-cell count of the total B-cell count by immunophenotype is shown in Figure 2. Among the 1451 individuals with a CLL-like phenotype, the median of percent clonal B-cells was 1.9% (range, 0.1%-100%) and 52 were HC-MBL. The 92 individuals with an atypical clone had a median of percent clonal B cells of 14.3% (range, 0.2%-100%), and 10 were high-count. Among the 257 individuals with a non–CLL-like immunophenotype, the median was 28.2% clonal B cells (range 1.0%-100%), and 31 individuals were HC-MBL.
Boxplots showing the distribution of percent clonal B-cells for each immunophenotype. Distribution: n = 1451 CLL-like MBL clones, n = 92 atypical MBL clones, and n = 257 non–CLL-like MBL clones. Eighty-five individuals had >1 immunophenotype and thus were included in more than one group.
Boxplots showing the distribution of percent clonal B-cells for each immunophenotype. Distribution: n = 1451 CLL-like MBL clones, n = 92 atypical MBL clones, and n = 257 non–CLL-like MBL clones. Eighty-five individuals had >1 immunophenotype and thus were included in more than one group.
Association with incident hematological cancers
We next evaluated the association of MBL with incident hematological malignancies. Among the 4010 individuals in the discovery cohort, 1651 individuals (214 MBLs and 1437 controls) were residents of the 27-country region surrounding Mayo Clinic at the time of sample collection and had robust follow-up for the development of hematological cancers. The median follow-up time in this group was 10.0 years. A total of 31 individuals (both controls and MBLs) developed incident hematological cancer with two-thirds of these cancers diagnosed as lymphoid malignancies (supplemental Table 1). Three of 31 individuals who developed hematological cancer progressed to CLL, and 2 of these 3 individuals had CLL-like MBL 5.9 and 10 years before CLL. We found that 13 of 214 individuals with MBL developed incident hematological cancer, and 18 of 1437 controls developed incident hematological cancer (supplemental Table 1; Figure 3A). The age- and sex-adjusted 5-year and 10-year cumulative incidence of any hematological cancer among MBLs was 3.3% and 5.8%, respectively, whereas among controls, the cumulative incidence was 0.8% and 1.1%, respectively (Figure 3A). After adjusting for age and sex, MBL was associated with a 3.6-fold higher risk of incident hematological cancer compared to controls (95% CI, 1.7-7.7; P < .001). This association is driven by incident lymphoid malignancies. Of 13 individuals with MBL who progressed to hematological cancer, all but one of the hematological cancers was lymphoid (supplemental Table 1). When we evaluated the association of MBL with risk of incident lymphoid malignancies compared to controls, we observed a 7.7-fold increased risk (95% CI, 3.1-19.2; P < .001) (Figure 3B). When we compared the subset of individuals with HC-MBL (n = 15) to controls, the risk of progression to any lymphoid malignancy was 74-fold (95% CI, 22.3-245.9; P < .001). When we compared individuals with LC-MBL (n = 199) to controls, the risk of progression to any lymphoid malignancy was 4.3 (95% CI, 1.4-12.7; P = .009). When we compared the subset to those MBL with CLL-like or atypical immunophenotype MBL (n = 193), the risk for lymphoid malignancy was 6.8 (95% CI, 2.6-18.1; P < .001).
Cumulative incidence of cancers adjusted for competing risk of death. Cumulative incidence of hematological cancers (A), cumulative incidence of lymphoid cancers (B).
Cumulative incidence of cancers adjusted for competing risk of death. Cumulative incidence of hematological cancers (A), cumulative incidence of lymphoid cancers (B).
Association with OS
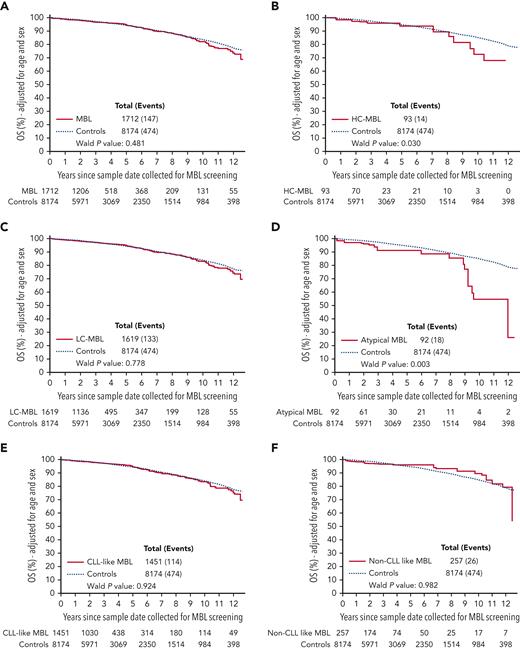
Next, we followed 9866 individuals for OS. The median follow-up time from the MBL screening assay was 34.4 months (range, 0-151.5 months), and 621 individuals subsequently died. The age- and sex-adjusted 5-year and 10-year OS among MBLs was 94.7% and 82.2%, respectively, and among controls, the estimates were 94.3% and 83.8%, respectively. We did not observe a significant difference in OS between individuals with MBL vs controls (HR, 1.1; 95% CI, 0.9-1.3; P = .48) (Figure 4A), nor when we stratified the cohort by age (<65 or age ≥65 years) (supplemental Figure 2), by sex (supplemental Figure 3), or by study phase (the discovery cohort had a median of 10 years of follow-up and the validation cohort had a median of 3 years) (supplemental Figure 4). When we stratified by the size of the clone, individuals with any HC-MBL had a significantly shorter OS compared controls (HR, 1.8; 95% CI, 1.1-3.1; P = .033) (Figure 4B), whereas those with LC-MBL did not significantly differ from controls (HR, 1.0; 95% CI, 0.8-1.3; P = .78) (Figure 4C). When we stratified by MBL immunophenotype, individuals with atypical MBL had a shorter OS compared to controls (HR, 2.0; 95% CI, 1.3-3.3; P = .003) (Figure 4D); however, individuals with CLL-like MBL or non–CLL-like MBL had a similar OS as that of controls (all P > .05) (Figure 4E-F, respectively).
Overall survival. OS adjusted for age and sex between MBL and controls (A), between HC-MBL,and controls (B), between LC-MBL and controls (C), between atypical MBL and controls (D), between CLL-like MBL and controls (E), and between non–CLL-like MBL and controls (F).
Overall survival. OS adjusted for age and sex between MBL and controls (A), between HC-MBL,and controls (B), between LC-MBL and controls (C), between atypical MBL and controls (D), between CLL-like MBL and controls (E), and between non–CLL-like MBL and controls (F).
Incidence and natural history of MBL
A total of 626 individuals from our discovery cohort returned a blood sample for a second MBL screening a median of 9.9 years (range, 4.8-12.0 years) after the initial sample. Among these, we evaluated MBL incidence (for those who initially screened negative for MBL) and persistence of the MBL clone (among those who initially screened positive for MBL).
MBL incidence
A total of 566 individuals who had no evidence of MBL at baseline screening provided a subsequent blood sample for sequential MBL screening evaluation a median of 9.9 years from initial screening (range, 4.8-10.9 years). Of these, 3 had too few cells to evaluate MBL, 496 still had no evidence of MBL, and 67 (13%) individuals subsequently developed MBL. Similar to prevalent MBL, the proportion of incident MBL was higher among males (18%) compared to females (9%). The median age of incident MBL was 74 years (range, 54-92 years). The incidence increased with age ranging from 4% in those between 40 to 59 years of age to 18% in those 80 years of age or older (supplemental Figure 5). CLL-like immunophenotype was most common (n = 58 individuals), followed by non–CLL-like (n = 12 individuals), then atypical (n = 1) with 4 individuals having more than 1 immunophenotypic clone. Among the 58 CLL-like phenotype clones, the median percent clonal B-cell count was 1.3% (range, 0.2%-93.6%) compared to 1.9% (range, 0.1%-100%) among the original prevalent CLL-like MBLs. Among these incident MBL cases, 3 individuals progressed from being a control at baseline to HC-MBL (1 with CLL-like MBL and 2 with non–CLL-like MBL) at the second evaluation.
MBL clonal progression
We had 58 individuals who had a baseline MBL clone and who had subsequent MBL screening evaluation. Of these, we observed that the MBL clone persisted in 54 (93%) individuals at follow-up. The CLL-like MBL clone persisted in 48 (94%) of the 51 individuals with a CLL-like MBL clone at baseline evaluation, whereas the remaining 3 individuals with CLL-like MBL at baseline had no evidence of MBL at the second evaluation. There was 1 individual with atypical MBL at baseline that did not persist at follow-up; among 6 individuals with non–CLL-like phenotype at baseline, all persisted at follow-up evaluations. We observed heterogeneity in progression in percent clonal B-cell counts among 48 individuals with CLL-like MBL at both time points (Figure 5). Of these 48 individuals, 27 had percent clonal B-cell counts less than 10% at both time points (Figure 5A) showing little to no progression in their clone; whereas 15 individuals showed clear progression at the time of the second sample (Figure 5B).
Change in clonal counts among 48 individuals with CLL-like MBL at both baseline and follow-up evaluation. (A) Individuals (n = 27) whose percent clonal B-cell count was <10% both time points. (B) Individuals (n = 21) who had at least one time point whose percent clonal B-cell count was >10%.
Change in clonal counts among 48 individuals with CLL-like MBL at both baseline and follow-up evaluation. (A) Individuals (n = 27) whose percent clonal B-cell count was <10% both time points. (B) Individuals (n = 21) who had at least one time point whose percent clonal B-cell count was >10%.
Discussion
In a large screening cohort of more than 10 000 individuals from the Mayo Clinic Biobank, we found that MBL was common (17%) and that individuals with MBL progressed to hematological malignancies (including CLL, diffuse large B-cell lymphoma, and mantle cell lymphoma) at an estimated rate of ∼0.5%/year. Compared to controls, individuals with HC-MBL had a significantly higher rate of progression to lymphoid malignancies vs LC-MBL (a 75-fold increased risk vs 4.3-fold increased risk, respectively). In a cohort of individuals with a strong family history of CLL from the Genetic Epidemiology of CLL consortium, we reported the rate of progression from LC-MBL to CLL to be 1.1% per year.1 The higher rate of progression in the familial cohort is expected due to the strong inherited component of the risk of CLL.21,22 Collectively, across these studies, we see an emerging gradient in the risk of progression to hematological cancers among individuals with MBL ranging from those with no family history who have LC-MBL, to those with family history who have LC-MBL, to those who have HC-MBL on screening. Herein, we report the prevalence of HC-MBL from screening to be 5%, with CLL-like being the most common immunophenotype. It is unknown whether there is a difference in the rate of progression between individuals with HC-MBL identified clinically or through screening. Our MBL screening was performed using frozen samples enriched for mononuclear cells. This likely contributed to a higher sensitivity of our screening assay compared to prior studies resulting in a higher prevalence of MBL overall. This may impact comparison of the prevalence to other studies.
Despite MBL being common, the OS among those with and without MBL were similar. We also did not observe any significant difference in OS when we stratified the cohort by age, sex, or phase of study (discovery or validation cohort). Further, similar survival was observed among 1619 individuals with LC-MBL compared to 8174 controls. This latter result contrasts a small prior study of 65 LC-MBLs and 290 controls who reported significantly shorter survival among the those with MBL.15 Given the size of our study and length of follow-up, the smaller study may be considered to have false-positive results. When we created subsets of individuals with MBL by the size of the clone or by the immunophenotype, we observed significantly shorter survival rates compared to controls. Specifically, individuals with an atypical phenotype MBL and individuals with HC-MBL had reduced OS compared to controls. In the present study, our numbers are too small to evaluate OS in the HC-MBLs by immunophenotype status. In a separate study, we recently reported longer OS among a cohort of 415 individuals who were predominantly HC-MBL with CLL-like phenotype ascertained in the clinic compared to age- and sex-matched controls.23 Individuals with MBL identified in the clinic may have greater clinical surveillance and enhanced counselling about risk of infections, vaccinations, and risk of malignancies (including skin cancers).
It has been established from multiple studies that individuals with CLL would have passed through an MBL phase before developing CLL.1,24,25 However, not all individuals with MBL will progress to CLL. Similar to prior studies,15,26 the MBL clone persisted over time in the majority of individuals we studied. We did observe a number of individuals with CLL-like MBL who progressed in their clonal size similar to prior studies.15 Prognostic factors for progression among individuals with MBL include the CLL international prognostic index23 and the number of recurrently mutated CLL driver genes.27 In many cases, the lymphoid malignancy that eventually occurred among individuals with MBL had an immunophenotype distinct from the pre-existing MBL clone. This suggests that identification of LC-MBL may be a marker for future risk of lymphoid malignancy but that, in many cases, these lymphoid malignancies may represent origination of an independent clonal process rather than “progression” of the LC-MBL clone.
Several strengths of our study include the large cohort for MBL screening, all of whom were known not to have any prior hematological cancer at the time of MBL screening and who had no knowledge of the MBL screening results; the ability to accurately evaluate the incidence of hematological cancers obtained from medical record linkage system with 10 years of median follow-up time; and the high-sensitive flow cytometry approach conducted by skilled technicians with extensive experience as well as supervision by an expert hematopathologist. Our cohort had unequal proportions of females (61%) and males (39%) screened for MBL. This higher frequency of females in our cohort reflects the higher frequency of females (59%) who participated in the Mayo Clinic Biobank from which our MBL screening cohort was derived. Although we screened more females than males, we observed a higher prevalence of MBL in males compared to females reflecting what is observed in CLL and what has been previously reported in some MBL screening studies28 but not all.1 Several limitations should be noted. Given that we only had 31 individuals who developed hematological cancers, we were not able to evaluate progression to specific lymphoma subtypes nor were we able to evaluate progression stratified by both immunophenotype and size of clone.
In summary, in this large cohort study, we observed similar survival among individuals with and without LC-MBL. This fact is reassuring given the high prevalence of MBL, a condition that is estimated to be present in 8 to 10 million adults older than the age of 40 years in the United States. Nonetheless, individuals with LC-MBL were at increased risk for future lymphoid malignancy. When considered with our prior findings that individuals with LC-MBL have an increased risk of serious infections,14 accumulating evidence indicates that there are clinical consequences to LC-MBL. Additional research is needed to understand the factors that predispose to MBL. Currently, age, sex, and inherited variants are risk factors variants.18,29-31 Further work is also needed to understand the variables that influence development of lymphoid malignancy and other potential health implications among individuals with MBL.
Acknowledgments
The authors thank the individuals from the Mayo Clinic Biobank for their contributions and continuing participation. This work would not be done without their valuable time and effort.
This work was supported by the National Institutes of Health (NIH), National Institute on Aging grant R01 AG58266 and NIH National Cancer Institute grants R01 CA200703, and P50 CA097274. The Mayo Clinic Center for Individualized Medicine provided the Mayo Clinic Biobank materials.
Authorship
Contribution: Concept and study design was performed by S.L.S. and T.D.S.; acquisition of data was performed by T.D.S., S.A.P., S.J.A., C.E.L., J.E.O., J.R.C., and S.L.S.; analysis of data was performed by S.J.A., K.G.R., and S.L.S.; interpretation of data was performed by all authors; the manuscript was drafted by S.L.S. and T.D.S.; and the manuscript was reviewed by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan L. Slager, Divisions of Hematology and Computational Biology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; e-mail: slager@mayo.edu.
References
Author notes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal