Abstract
Since its initial identification in 1992 as a possible class 1 cell-surface receptor without a known parent ligand, receptor tyrosine kinase–like orphan receptor 1 (ROR1) has stimulated research, which has made apparent its significance in embryonic development and cancer. Chronic lymphocytic leukemia (CLL) was the first malignancy found to have distinctive expression of ROR1, which can help distinguish leukemia cells from most noncancer cells. Aside from its potential utility as a diagnostic marker or target for therapy, ROR1 also factors in the pathophysiology of CLL. This review is a report of the studies that have elucidated the expression, biology, and evolving strategies for targeting ROR1 that hold promise for improving the therapy of patients with CLL or other ROR1-expressing malignancies.
Receptor tyrosine kinase–like orphan receptors
ROR1 was identified using a polymerase chain reaction–based approach to amplify genes that could encode proteins with tyrosine kinase–like domains.1 This gene, along with another sharing 58% sequence homology, were each deduced to encode a cell-surface protein with an extracellular immunoglobulinlike domain, a cysteine-rich domain, a Kringle domain, and an intracellular tyrosine kinase–like domain and 2 serine/threonine-rich sections flanking a proline-rich domain (PRD; Figure 1). These proteins were considered likely to encode cell-surface receptors related to tropomyosin receptor kinase. Because their ligand(s) were unknown, they were termed “orphan receptors” and called receptor tyrosine kinase–like orphan receptors 1 and 2 (ROR1 and ROR2).
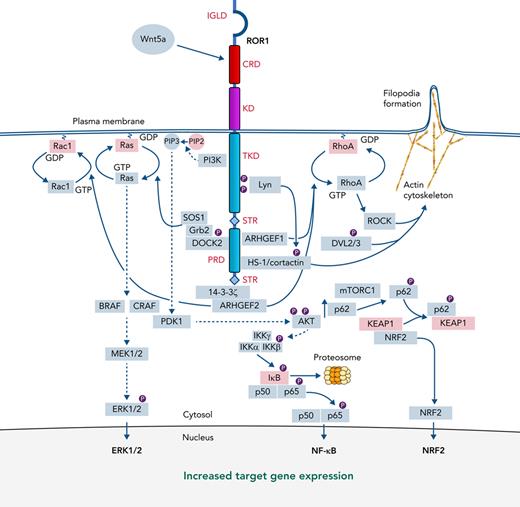
ROR1 signaling. The structure of ROR1 is provided with each of the various domains labeled in red font to the right or left of the ROR1 stick figure, marking the extracellular immunoglobulinlike domain, CRD, and Kringle domain and the intracellular tyrosine kinase–like domain, the serine/threonine-rich domains, and the proline-rich domain (PRD). The binding of Wnt5a to the CRD can trigger activation of ROR1 signaling with recruitment and activation of cytosolic accessory molecules in green rectangles via defined (solid arrows) or speculated (dashed arrows) signaling pathways, culminating in the expression of ERK1/2, NF-kB, and NRF2 target genes in the nucleus. Red rectangles delineate inactive or inhibitory signaling molecules. Small circles labeled P represent protein phosphorylation.
ROR1 signaling. The structure of ROR1 is provided with each of the various domains labeled in red font to the right or left of the ROR1 stick figure, marking the extracellular immunoglobulinlike domain, CRD, and Kringle domain and the intracellular tyrosine kinase–like domain, the serine/threonine-rich domains, and the proline-rich domain (PRD). The binding of Wnt5a to the CRD can trigger activation of ROR1 signaling with recruitment and activation of cytosolic accessory molecules in green rectangles via defined (solid arrows) or speculated (dashed arrows) signaling pathways, culminating in the expression of ERK1/2, NF-kB, and NRF2 target genes in the nucleus. Red rectangles delineate inactive or inhibitory signaling molecules. Small circles labeled P represent protein phosphorylation.
ROR1 has a high degree of protein-sequence conservation among different species; for example, mouse and human ROR1 share 97% amino acid sequence homology.2 Even Drosophila melanogaster possesses a receptor tyrosine kinase–like protein called Dror, which has 36% and 61% amino acid sequence identity with human ROR1 in its extracellular domain and tyrosine kinase–like domain, respectively.3 Also conserved is the developmental expression of ROR1 and ROR2 in embryogenesis, in which these proteins apparently function in organ development.4,5 Mice made deficient in ROR1 have developmental abnormalities and impaired viability.6,7 Mice made deficient in both ROR1 and ROR2 have even more profound defects8 that mimic those of mice made deficient in the noncanonical wingless-related integration site (Wnt) factor Wnt5a.9
Expression of ROR1 attenuates during fetal maturation,4 becoming virtually negligible on most postpartum tissues,10 except on early B-cell precursors, morphologically known as hematogones.11 However, antibodies apparently specific for ROR1 also can react with cells in the parathyroid, pancreatic islets, adipose tissue, or gastrointestinal tract of adults.12,13 Such tissue cross-reactivity is less apparent for some anti-ROR1 monoclonal antibodies (mAbs),14 suggesting that some tissues express cross-reactive proteins other than ROR1 or have peculiar ROR1 posttranslational modifications that react with only some anti-ROR1 antibodies.
Ending its status as an orphan receptor, we demonstrated that ROR1 was a receptor for Wnt5a.10 Wnt5a is considered a noncanonical Wnt factor, because it does not activate the canonical Wnt-signaling pathway, which induces nuclear translocation of the transcription factor β-catenin.15,16 The CRD has structural similarities with the CRDs of other Wnt receptors that bind amphipathic Wnt factors.17 Coimmuneprecipitation studies have demonstrated that the extracellular domain of ROR1 binds Wnt5a, but not the canonical Wnt factor Wnt3a.10 Furthermore, Wnt5a stimulation of cells made to express ROR1 does not activate the canonical Wnt-signaling pathway, but rather activates NF-κB.10
ROR1 in chronic lymphocytic leukemia
Gene expression profiling revealed the chronic lymphocytic leukemia (CLL) cells of different patients shared a common gene expression signature, which included ROR1.18 That CLL cells distinctively expressed surface ROR1 was indicated by studies evaluating the sera of patients treated with autologous leukemia cells made to express CD154 to induce antileukemia immune responses.19 The sera of some treated patients developed IgG autoantibodies that bound to CLL cells, but not to normal B lymphocytes; these autoantibodies were found to bind ROR1.10,19 Anti-ROR1 autoantibodies subsequently were found in sera of some patients treated with lenalidomide,20 as well as in the sera of a few untreated patients, particularly those with nonprogressive disease.21
Studies with ROR1-specific mAbs have confirmed that surface ROR1 is expressed by CLL cells, but not by normal B lymphocytes,10,22,23 including normal CD5 B cells.10 Baskar and colleagues estimated that the number of surface ROR1 molecules per cell ranges from 1000 to 10 000,23 which is comparable to that of CD20 (eg, 6000-14 000).24 Studies showed by quantitative immune fluorescence that the number of equivalent fluorescence molecules per CLL cell ranged from 2000 to 11 000,25 except on the CLL cells of fewer than 5% patients who lacked detectable ROR1.26
Because of its distinctive expression, ROR1 was proposed as a diagnostic marker for CLL.27 Studies showed that fluorescence-tagged anti-ROR1 mAbs could readily distinguish CLL cells from normal lymphocytes by flow cytometry10,27-29 and could help detect minimal residual disease after therapy.30,31 ROR1 also was detected on the CD5+ B cells of some adults with monoclonal B-cell lymphocytosis,32 a condition that can presage CLL.33 These studies established the utility of assays for ROR1, which currently is among the 6 markers recommended by the European Research Initiative on CLL and the European Society For Clinical Cell Analysis for refining the diagnosis of CLL.28
ROR1 in other cancers
Selective postpartum expression of ROR1 is not confined to CLL, but has been identified in other cancers, particularly those with high-grade histology and/or high metastatic potential.34-37 Among hematologic malignancies, surface expression of ROR1 is most prominent on CLL, hairy cell leukemia, and mantle cell lymphoma (MCL).38,39 Prominent expression of ROR1 can be detected on the neoplastic cells of patients with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), myeloma, acute lymphocytic leukemia (ALL), and myeloid leukemias.11,38-40 Cases of DLBCL associated with Richter transformation can express ROR1.41 Although the proportions of ROR1+ cells in CLL, hairy cell leukemia, or MCL appear higher than in DLBCL, and higher still than in FL, the prevalence of ROR1 on other leukemias, lymphomas, and solid-tissue cancers indicates that sole detection ROR1 is not diagnostic of CLL.38
High-level ROR1 associates with aggressive CLL
The level of ROR1 on CLL has clinical significance. In a study of 20 patients, Daneshmanesh and colleagues found patients with CLL cells that had high levels of ROR1 were more likely to have progressive disease than were those with CLL with low levels of ROR1.42 Similarly, transgenic B-cell expression of ROR1 accelerates development of leukemia and shortens survival of Eμ-TCL1-transgenic mice, which develop a CD5 B-cell leukemia that mimics CLL.43 This association between high-level expression of ROR1 and adverse outcome was established in a subsequent study of 1568 patients by the CLL Research Consortium.26 Cases were randomly assigned to a training set or validation set of 797 or 771 patients, respectively. A threshold for high-level surface ROR1 (ROR1-Hi) was established in the training set that could distinguish cases into ROR1-Hi vs ROR1-Lo subgroups that differed significantly in their median time from diagnosis to initial treatment, or treatment-free survival (TFS). This threshold subsequently was found to correspond to ∼6.2 × 103 immune fluorescence molecules per cell.25 For both the training set and validation set, this threshold could distinguish patients that differed significantly in their median TFS; patients in the ROR1-Hi subset also had shorter overall survival (OS) than the patients in the ROR1-Lo subgroup.26
High-level ROR1 significantly associated with other adverse prognostic markers, such as the expression of unmutated immunoglobulin heavy chain variable region (U-IGHV) genes (P < .001) in both training and validation cohorts.26 However, many patients with CLL cells that used mutated IGHV (M-IGHV) also had high-level expression of ROR1; the association between high-level ROR1 with relatively adverse outcome was particularly apparent among such patients. High-level ROR1 also associated with a significantly shorter median TFS and OS among patients with CLL that used U-IGHV.26 Nevertheless, patients with CLL cells that used U-IGHV, but had low levels of ROR1, still had a shorter median TFS and OS, than did patients with CLL cells that used M-IGHV, but had high ROR1, indicating that high-level ROR1 cannot solely account for the relatively aggressive disease observed for patients with CLL cells that use U-IGHV.44-46
Wnt5a, the ligand for ROR1,10 is also expressed in CLL. One study found that some patients had CLL cells that expressed Wnt5a, suggesting that Wnt5a functions as an autocrine.47 Moreover, those with Wnt5a+CLL cells apparently had a shorter TFS than patients with CLL lacking Wnt5a.47 However, 85% or 50% of the respective patients that used M-IGHV or U-IGHV had CLL cells that lacked detectable Wnt5a.47 In contrast, virtually all patients have high plasma levels of Wnt5a, which is not detected in age-matched healthy adults.25,48,49 Moreover, monocytes and macrophages (eg, nurselike cells),50 can express high levels of Wnt5a, which may stimulate leukemia cells nestled within the leukemia microenvironment.49,51
Factors influencing expression of ROR1
Signal transducer and activator of transcription 3 (STAT3)
The promoter for ROR1 harbors γ-interferon–activation sequence elements that can be activated by STAT3.52 Chromatin immunoprecipitation assays confirmed that serine-phosphorylated STAT3 binds the ROR1 promoter at 2 sites, which were identified by electromobility shift assays.52 Finally, STAT3 activation can enhance leukemia-cell expression of ROR1, but is not sufficient to induce it on cells lacking ROR1.
Similarly, STAT3 activation may enhance expression of Wnt5a. Rozovski and colleagues identified STAT3-binding sites close to the gene encoding Wnt5a (WNT5A); chromatin immunoprecipitation and electromobility shift assays demonstrated that phosphorylated STAT3 could bind to the WNT5A promoter.53 Moreover, silencing STAT3 reduced expression of Wnt5a and enhanced leukemia-cell apoptosis.53 However, immunoblot analyses showed an apparent inverse correlation between the levels of Wnt5a and ROR1 among samples, indicating that factors other than STAT3 influence expression ROR1 and/or Wnt5a in CLL cells.
MicroRNA
ROR1 also may be influenced by certain micro-RNAs (miRs).54 Specifically, miR-15a and miR-16-1, which frequently are deleted in CLL with del(13q),55,56 each target ROR1, in addition to targeting BCL2,57 which encodes the antiapoptotic protein BCL2. CLL cells with undetectable levels of miR15a/16-1 commonly express high levels of ROR1 and BCL2.54 The relatively high levels of BCL2 expressed by such cells may enhance their resistance to the BCL2 antagonist venetoclax.
Although miR15a/16-1 can downregulate ROR1, high-level expression of ROR1 is not restricted to CLL cells with del(13q). Instead, high-level ROR1 is significantly more prevalent among cases with adverse prognostic genetic features (eg, expression of U-IGHV).26
Posttranslational modifications and degradation
Posttranslational modifications of ROR1 govern its surface expression and degradation. The polypeptide sequence of ROR1 has a predicted molecular size of 104 kDa.58 However, nascent ROR1 undergoes N-linked glycosylation, allowing for its transport to and expression on the plasma membrane as a glycoprotein of ≈130 kDa.59 Inhibition of glycosylation interferes with the cell surface expression of ROR1.
ROR1 also undergoes phosphorylation,60 which may enhance its proclivity to undergo polyubiquitination and proteasomal degradation. Indeed, ubiquitinated forms of ROR1 can be detected in CLL cells.59 The Src family tyrosine-kinase Lyn can phosphorylate tyrosine (Y) residues within the tyrosine kinase–like domain of ROR1: Y645 and Y646,61 which can be phosphorylated also by Src.62 Lyn-phosphorylated ROR1 can recruit c-Casitas B-lineage lymphoma, an E3 ligase that binds to phosphotyrosine motifs of proteins it targets for degradation.63-65 However, ROR1 also has a binding motif (ELHHPNIV) for heat shock protein 90, which can bind and protect phosphorylated ROR1 from proteasomal degradation.66 Consistent with this notion, treatment of CLL cells with an Hsp90 inhibitor, 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), reduces expression of ROR1 and ROR1 signaling.66
ROR1 signaling
Freshly isolated CLL cells typically have active ROR1 signaling, possibly reflecting stimulation by Wnt5a in the plasma.25,48,49,51 Short-term culture of leukemia cells in serum-free media deprives CLL cells of Wnt5a, allowing for study of Wnt5a-induced changes in ROR1+ CLL cells that can be inhibited by silencing ROR1,67 or by anti-ROR1 mAbs, such as UC-961 (cirmtuzumab, but now designated as zilovertamab).14 Although Wnt5a can induce ROR1 to hetero-oligomerize with ROR2,48,68 such hetero-oligomerization is not necessary for ROR1 signaling, which can occur in cells that lack ROR2.
Initial studies found that Wnt5a binds ROR1 and induces signaling in human embryonic kidney 293 (HEK293) cells transfected to express ROR1, leading to activation of NF-κB, but not the canonical Wnt signaling pathway mediated by β-catenin.10 Activation of NF-κB appears mediated by protein kinase B (AKT), which may be phosphorylated after recruitment of phosphoinositide 3-kinase, conversion of phosphatidylinositol (4,5)-bisphosphate (PIP2) to PIP3, and activation of 3-phosphoinositide–dependent protein kinase 1 (Figure 1).35,69 A key downstream target for NF-κB is p62, which is an autophagy and signaling adaptor that can enhance signaling by mechanistic target of rapamycin complex 1 (mTORC1). ROR1 signaling associates with accumulation of phosphorylated p62,70 which binds the Kelch-like ECH-associated protein 1 (KEAP1), thereby inhibiting KEAP1 from sequestering nuclear factor-erythroid factor 2–related factor 2 (NRF2; Figure 1).71 NRF2 is a transcription factor that influences the expression of >1000 genes encoding glutathione and thioredoxin antioxidant systems, as well as enzymes involved in the regeneration of reduced nicotinamide adenine dinucleotide phosphate and the detoxification of exogenous and endogenous products,72 thereby enhancing resistance to anticancer drugs that induce reactive oxygen species.73 On the other hand, inhibition of ROR1 signaling by zilovertamab, can reduce expression of p62 and target genes induced by activation of NF-κB, mTORC1, and NRF2,49,70,74 thereby potentially enhancing the cytotoxicity of such drugs.
Wnt5a also can induce ROR1 to recruit guanine exchange factors, which can effect activation of ρ/Rac GTPases.48 Stimulation of ROR1 by Wnt5a induces recruitment of 14-3-3ζ,75 a highly conserved phosphoserine-binding protein implicated in the pathogenesis of multiple cancers. Recruitment of 14-3-3ζ is dependent on a serine at position 857 of ROR1, within an identified 14-3-3 binding motif (RSPS857SAS) in the C-terminal serine/threonine–rich section (Figure 1).75 ROR1-14-3-3ζ can recruit the ρ/Rac guanine nucleotide exchange factor 2, which can activate RhoA and Rac1. Wnt5a also can induce ROR1 to recruit dedicator of cytokinesis 2 (DOCK2), which is another Rac-specific guanine-nucleotide–exchange factor that can activate Rac1/2.76 DOCK2 binds to activated ROR1 at an SH3-binding site dependent on the proline at position 808 in the PRD of ROR1 (Figure 1).76 Wnt5a-induced activation and recruitment of DOCK2 to ROR1 also conscripts the growth factor receptor-bound protein 2 (Grb2) and the Ras-guanine exchange factor, son of sevenless homolog 1 (Sos1),77 forming a complex that can activate Ras, which then can trigger activation of the MAPK kinase cascade. This process culminates in the phosphorylation and activation of extracellular signal-regulated kinases (ERK), ERK1 and ERK2 (ERK1/2; Figure 1), to enhance leukemia-cell proliferation.74
Wnt5a also can cause ROR1 to recruit hematopoietic lineage–specific protein 1 (HS-1) and/or cortactin, which are Lyn substrates that then undergo tyrosine phosphorylation.25,78,79 The complex of HS-1/cortactin links ROR1 to the actin cytoskeleton, thereby promoting planar cell polarity, filopodia formation, and chemokine-directed migration.78 HS-1 and cortactin are recruited to the same SH3-binding site, which is dependent on the proline at position 841 within the ROR1 PRD.78,79 Breast cancer cells, which lack HS-1, recruit only cortactin to this SH3-binding site.80 The enhanced proclivity to form metastases of breast cancer cells that express ROR1 is significantly impaired when the same tumor cells are made to express a mutant ROR1 with a proline-to-alanine substitution at position 808,80 thereby highlighting the importance of this SH3-binding site to the migration and invasion of ROR1-expressing cancer cells.
Expression of ROR1 and Wnt5a during embryogenesis associates with phosphorylation of dishevelled 2 (DVL2),9 one of three DVL proteins found in CLL cells at levels that are higher than those found in lymphocytes of healthy adults.81 Khan and colleagues reported that DVL2 and DVL3 were constitutively phosphorylated in CLL cells, the level of which could be reduced by treatment with an anti-ROR1 mAb,81 suggesting that ROR1 signaling also involves serine/threonine phosphorylation of DVL2 and/or DVL3, which in turn may contribute to activation of RhoA and enhanced cell migration.
Wnt5a also can induce leukemia-cell phosphorylation of STAT3 (pSTAT3), which could be blocked by zilovertamab or by neutralizing antibodies specific for Wnt5a.49 However, time course studies revealed that Wnt5a required more than 3 hours to induce pSTAT3, which peaked several hours later, suggesting that Wnt5a indirectly induced pSTAT3. In contrast, Wnt5a induced activation of NF-κB within 30 minutes, resulting in leukemia cell expression of NF-κB target genes, including those encoding proinflammatory cytokines/chemokines (eg, interleukin 6 [IL-6], IL-8, CCL2, CCL3, CCL4, and CXCL1), which in turn induced pSTAT3 in unstimulated leukemia cells, particularly IL-6.49 Wnt5a-induced CLL-cell expression of proinflammatory cytokines/chemokines could be inhibited by treatment of CLL cells with zilovertamab or drugs that inhibit NF-κB, such as BAY 11-7082.49 However, zilovertamab could not inhibit autocrine IL-6 activation of pSTAT3, which instead was inhibited by tocilizumab, a mAb specific for the IL-6-receptor.49 On the other hand, treatment of patients with zilovertamab reduced plasma levels of IL-6 and leukemia-cell activation of NF-κB and STAT3, providing evidence that this circuitry operates in vivo,25,49 potentially contributing to constitutional symptoms experienced by some patients with CLL.
ROR1-signaling pathways most likely are influenced by crosstalk with other cell-surface receptors and available cytosolic accessory molecules. For example, ligation of surface immunoglobulin can induce activation of Lyn,82 which may influence ROR1 signaling.61 On the other hand, the antileukemia activity of BCR-signaling inhibitors may be mitigated by active ROR1 signaling,83-86 and vice versa. Also, some receptors and accessory proteins, such as HS-1 or DOCK2, are predominately expressed by hematopoietic cells; the interaction of ROR1 with alternative cytosolic accessory molecules expressed in other tissue types may alter the tenor of ROR1 signaling in nonhematopoietic malignancies.80,87
Targeting ROR1 for therapy
Anti-ROR1 mAbs
The distinctive expression of ROR1 on CLL cells may be targeted for clinical benefit.88 Daneshmanesh and colleagues generated mAbs specific for any one of the 3 extracellular domains of ROR1; 1 mAb (3B8) bound the Ig-domain, 2 (1C11 and 1D8) each recognized the CRD domain, and another 2 (4C10 and 4A7) each recognized the Kringle domain.42 They found that each could directly induce apoptosis of CLL cells without complement or effector cells.42 Yang and colleagues developed chimeric rabbit/human IgG1 antibodies that were specific for one or more extracellular domains of ROR1, designated R11, R12, or Y31.89 However, none of these mAbs induced direct or complement-dependent cytotoxicity; only 1, R12, could direct antibody-dependent cell-medicated cytotoxicity. Similarly, Baskar and colleagues generated 2 mouse mAbs (2A2 and 2D11) that recognized N-terminal epitopes of ROR1.90 These mAbs also were not cytotoxic for ROR1+ leukemia cells unless they were coupled with an exotoxin.90
Zilovertamab is a humanized IgG1 mAb selected for its ability to block ROR1 signaling by binding the same epitope on ROR1 as recognized by a prototypic anti-ROR1 mAb, D10, which could inhibit engraftment of transgenic mouse leukemia B cells that expressed human ROR1.14,43 In a phase 1 study, zilovertamab had a plasma half-life of 32.4 days, with no discernable dose-limiting toxicity.25 Immunoblot and transcriptome analyses of isolated CLL cells revealed that the CLL cells of patients 1 month into therapy with zilovertamab had significantly lower levels of phosphorylated NF-κB p65 (pp65) and phosphorylated HS-1 and diminished expression of target genes induced by activation of NF-κB, mTORC1, NRF2, ERK1/2, RhoA, Rac1, and STAT3, than the CLL cells of the same patients collected before therapy.25,49,70,74 Moreover, posttreatment CLL cells had a highly significant reversal of gene-expression signatures associated with stemness and oncogenic dedifferentiation,91 which were observed in the leukemia cells of the same patients before therapy. Although each of the enrolled patients required therapy per International Workshop for CLL guidelines,92 the median time to next therapy of patients after completing therapy with 4 biweekly infusions of zilovertamab was 262 days (registered on https://www.clinicaltrials.gov as #NCT02222688).25
Anti-ROR1 mAbs and Bruton tyrosine kinase inhibitors
Some of the survival-signaling pathways induced by Wnt5a remain active in patients who undergo therapy with a Bruton tyrosine kinase inhibitor (BTKi), such as ibrutinib.84,88 Treatment with ibrutinib inhibits trafficking of CLL cells into lymphoid tissues, precluding cells from entering the protective leukemia-microenvironment.51 However, the plasma levels of Wnt5a remain relatively high in patients who undergo therapy.84 As such, Wnt5a stimulation of ROR1 signaling may transcend the leukemia microenvironment. Although ibrutinib can block BCR signaling by its capacity to inhibit BTK, ibrutinib is not able to inhibit Wnt5a-induced ROR1-dependent activation of ρ/Rac GTPases or NF-κB.84 Possibly in part for this reason, treatment of immune-deficient mice engrafted with CLL cells with ibrutinib and zilovertamab was more effective than treatment with either agent alone in clearing engrafted leukemia cells.84
A similar dynamic is apparent in MCL. Ibrutinib could not inhibit Wnt5a-induced activation of Rac1 or enhanced proliferation of MCL cells; such effects could be inhibited by zilovertamab or by shRNA-directed silencing of ROR1.85,86 Moreover, inhibition of ROR1 signaling lowered the levels of activated phosphoinositide 3-kinase and ERK1/2, and reduced the proliferative capacity of MCL cells resistant to BTKi,93 suggesting that concomitant treatment with BTKi and inhibitors of ROR1 may mitigate the risk of developing resistance to BTKi therapy.
Multi-institutional trials of zilovertamab and ibrutinib for treatment of patients with CLL or MCL demonstrated this combination was well tolerated and without unexpected toxicity (registered on https://www.clinicaltrials.gov as #NCT03088878). Encouraging high response rates and long progression-free survival times were observed in treated patients, including those with MCL or relapsed/refractory CLL.94
Anti-ROR1 mAbs and venetoclax
Results of studies suggest that targeting ROR1 may enhance the efficacy of venetoclax. CLL cells with high levels of ROR1 also appear to have high levels of BCL2; treatment of such cells with zilovertamab could enhance their sensitivity to venetoclax.54 Moreover, patients who failed to clear minimal residual disease with venetoclax were found to have CLL cells with high levels of ROR1 before therapy that subsequently increased after ≥1 year of therapy.95 The CLL cells evading therapy had upregulation of genes induced by activation of ERK1/2 and NF-κB, including BCL2L1, encoding the antiapoptotic protein BCL-XL. Moreover, treatment of primary CLL cells with Wnt5a enhanced their resistance to venetoclax, an effect that could be inhibited by zilovertamab.95 Similarly, silencing ROR1 with short hairpin RNA in MCL cells also could increase their sensitivity to venetoclax,85 indicating that agents that inhibit ROR1 signaling may complement BCL2-inhibitor therapy. A clinical trial is evaluating zilovertamab and venetoclax for consolidation therapy for patients with persistent CLL, despite a year or more of venetoclax therapy (registered on https://www.clinicaltrials.gov as #NCT04501939).
Antibody-drug conjugates
Anti-ROR1 antibody-drug conjugates (ADCs) can selectively deliver a toxin, regulatory RNA, or cytotoxic drug to ROR1-expressing tumor cells. Baskar and colleagues generated a ROR1-immunotoxin (called BT-1) consisting of truncated Pseudomonas exotoxin A (PE38) linked to the anti-ROR1 mAb 2A2.90 BT-1 induced apoptosis of ROR1-expressing MCL cells (EC50, 16 pM to 16 nM), but was nontoxic for ROR1− cell lines.90 Chiang and colleagues coupled an anti-ROR1 mAb with a regulatory miR, miR-29b; in vitro treatment of ROR1+ cells with this ADC downregulated miR-29 target genes, DNMT1 and DNMT3A, altered global DNA methylation, reduced SP1, and enhanced expression of p21.96 Mani and colleagues coupled an anti-ROR1 mAb to nanoparticles containing OSU-2S, a drug that induces activation of protein phosphatase 2A (PP2A) and phosphorylation and nuclear translocation of SHP1(S591); treatment of ROR1+ cells with this ADC-induced apoptosis.97 This group also generated an ADC that could deliver a toxic anthracycline derivative specifically to ROR1+ cells.98
Zilovertamab vedotin (UC-961ADC3) was generated by conjugating UC-961 (zilovertamab) with a tubulin-synthesis inhibitor, monomethyl auristatin E (MMAE), using linkers that allowed for intracellular release of MMAE, which is highly toxic to cells undergoing mitosis.99 UC-961ADC3 was selectively toxic only for ROR1+ tumor cell lines,99 which led to the development of zilovertamab vedotin (formerly VLS-101), an ADC composed of zilovertamab, a maleimidocaproyl-valine-citrulline-para-aminobenzoate linker, and MMAE, which has selective toxicity for ROR1+ tumor cells.100 This ADC caused marked tumor regression in immune-deficient mice engrafted with MCL cells from patients resistant to therapy with anti-CD19-CAR T cells, ibrutinib, and/or venetoclax,101 or DLBCL cells from patients with Richter transformation.41 In a phase 1 study involving heavily pretreated patients with relapsed or refractory MCL (n = 15), CLL (n = 7), DLBCL (n = 5), FL (n = 3), Richter transformation (n = 1), or marginal zone B-cell lymphoma (n = 1), zilovertamab vedotin elicited clinical responses in 7 of 15 patients with MCL (47%; 4 partial and 3 complete) and in 3 of 5 patients with DLBCL (60%; 1 partial and 2 complete); objective tumor responses were not observed among patients with other tumor types (registered on https://www.clinicaltrials.gov as #NCT03833180).102 The lack of activity in patients with indolent lymphomas could be secondary to the MMAE payload, which is cytotoxic for cells undergoing mitosis and hence is most active against tumors that have a high proliferation index. Clinical studies are evaluating the efficacy of zilovertamab vedotin in patients with relapsed/refractory DLBCL, either alone (registered on https://www.clinicaltrials.gov as #NCT05144841) or in combination with standard therapy (registered on https://www.clinicaltrials.gov as #NCT05139017), as well as in patients with solid tumors that express ROR1 (registered on https://www.clinicaltrials.gov as #NCT04504916).
Bispecific antibodies and bispecific T-cell engagers
Bispecific antibodies (BiAbs) are recombinant antibodies with 2 different heavy and light chain single-chain variable-region fragments (scFvs) attached to a common immunoglobulin constant region (Fc), whereas bispecific T-cell engagers (BiTes) are fusion proteins consisting of 2 different scFvs without an Fc. Either BiAbs or BiTes simultaneously can bind 2 different antigens or 2 different epitopes of the same antigen.
Qi and colleagues spliced each of several anti-ROR1 scFvs with an anti-CD3 scFv onto an aglycosylated IgG1 Fc to generate BiAbs that bound to ROR1 and CD3.103 They found that a BiAb with specificity for CD3 and a membrane-proximal ROR1 epitope derived from the mAb R11 was most effective in bridging T cells together with ROR1+ MCL cells so as to effect tumor-cell cytotoxicity.103 NVG-111 is an anti-ROR1/anti-CD3 BiTe that apparently could induce levels of cytokine release by T cell co-cultured with CD19+/ROR1+ lymphoma cells that were similar to that induced in such co-cultures by the BiTe blinatumomab.104,105 A phase 1/2 study of NVG-111 examined doses of NVG-111 from 0.3 to 360 μg/d via continuous infusion over 3 cycles (each 21 days on, 7 days off) in patients with relapsed/refractory CLL or MCL (registered on https://www.clinicaltrials.gov as #NCT04763083).105 It is hoped that such BiAbs or BiTes could have clinical activity in patients with CLL, as has been observed in patients with CD19+ ALL treated with blinatuomab.104
Small molecule inhibitors
ROR1 undergoes tyrosine phosphorylation in CLL cells,60,106 making it conceivable that ROR1 undergoes autophosphorylation in vivo, despite earlier findings that immunoprecipitated ROR1 had negligible intrinsic catalytic activity.107 Hojjat-Farsangi and colleagues screened a library of 110 000 small molecules and found 1 (KAN043983) that could cause dose-dependent inhibition of Wnt5a-induced ROR1 phosphorylation in CLL cells. Although it is not clear whether KAN043983 targets ROR1 directly, this drug can cause downregulation of BCL2, BCL-xL, MCL1, and BAX; cleavage of poly(ADP-ribose) polymerase and caspase 1/3; and apoptosis of primary CLL cells, but not of the blood mononuclear cells of healthy donors. Oral administration of this drug to immune-deficient mice engrafted with CLL cells reduced the number of CD45+/CD19+/ROR1+ cells in the spleen without systemic toxicity,106 stimulating interest in future clinical studies.
Anti-ROR1 CAR T cells
Hudecek and colleagues transduced T cells with a ROR1-specific CAR, enabling them to recognize and kill ROR1+ leukemia cells.12 These investigators constructed anti-ROR1-CARs from scFvs that bound ROR1 with different affinities and extracellular stalks that use IgG4-Fc spacer domains of different lengths. T cells expressing the optimal anti-ROR1-CAR were found as effective as anti-CD19–expressing CAR T-cells in clearing CD19+/ROR1+ JeKo-1 MCL xenografts in immunodeficient mice.108,109 Despite concern that the anti-ROR1 scFv used in this anti-ROR1 CAR could cross-react with some normal tissues, infusion of anti-ROR1 CAR T cells was well tolerated in nonhuman primates, which express a ROR1 that is virtually identical to human ROR1.110 Clinical studies are evaluating such anti-ROR1 CAR T cells in patients with CLL, MCL, ALL, non–small cell lung cancer, or triple-negative breast cancer (registered on https://www.clinicaltrials.gov as #NCT02706392).
Concluding remarks
Expression of ROR1 on CLL exemplifies cancer’s conscription of receptors required during embryogenesis for cell polarity, migration, and survival of cells that seed nascent organs. In so doing, cancer cells may enhance their fitness and capacity to seed other body sites with the pathologic caricatures of organ development known as metastases. The distinctive expression of such receptors, however, provides for developmentally restricted tumor-associated antigens. The study of ROR1 in CLL indicates that this antigen is not merely a marker that can help distinguish neoplastic from normal cells, but rather also is a surface receptor that can activate signaling pathways that enhance leukemia-cell migration, proliferation, and possible resistance to therapy. Research into strategies that target ROR1, however, may turn its selective advantage for neoplastic cells into cancer’s Achilles heel.
Acknowledgments
This work was supported by the University of California San Diego Foundation Blood Cancer Research Fund (BCRF) and National Institutes of Health, National Cancer Institute grant R01-CA236361.
Authorship
Contribution: T.J.K. wrote the manuscript and is solely responsible for its content.
Conflict-of-interest disclosure: Zilovertamab was developed by T.J.K. and his laboratory and licensed by the University of California to Oncternal+ Therapeutics, Inc, and VelosBio, Inc, which provided stock and research funding.
Correspondence: Thomas J. Kipps, 9310 Athena Circle, Suite 340, La Jolla, CA 92037; e-mail: tkipps@health.ucsd.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal