Key Points
Adolescents with mild HA had a 1.7-fold increase in FVIII:C with aerobic exercise, compared to a 1.9-fold increase seen with IN desmopressin.
Increase in FVIII:C with aerobic exercise was transient, whereas IN desmopressin was associated with more sustained improvement.
Abstract
Persons with mild hemophilia A (HA) may use intranasal desmopressin prior to sports participation. Desmopressin is expensive and can cause vomiting, headache, palpitation, and occasionally seizures. Our group has previously documented a 2.3-fold increase in factor VIII activity (FVIII:C) in adolescents with mild HA after moderate-intensity aerobic exercise. Herein, we report principal findings of a randomized trial of intranasal desmopressin vs a standardized, moderate-intensity aerobic exercise regimen in adolescents with mild HA. Our primary objective was to compare the change in FVIII:C associated with these 2 interventions. We also examined changes in hemostatic parameters arising from their sequential administration. The study was conducted simultaneously at the Hospital for Sick Children, Canada, and Nationwide Children’s Hospital, USA. Thirty-two eligible male adolescents (mean age ± standard deviation: 16.1 ± 2.6 years) with mild HA (mean baseline FVIII:C: 27.9% ± 18.4%) were randomized to 1 of 4 study arms (desmopressin followed by exercise, desmopressin alone, exercise followed by desmopressin, and exercise alone). Blood work was obtained at baseline and at 3 subsequent time-points. Participants randomized to exercise cycled on an ergometer for approximately 12 minutes, with the final 3 minutes at 85% of their predicted maximum heart rate. Standard weight-based dosing of desmopressin was used. Mean immediate increase in FVIII:C was 1.7-fold with exercise compared with 1.9-fold with desmopressin (noninferiority, P = .04). Exercise-induced improvement in hemostatic parameters including FVIII:C was brief compared with more sustained improvements seen with desmopressin. More than 60% of participants randomized to receive both exercise and desmopressin achieved normal (>50%) FVIII:C, 75 and 135 minutes into the study protocol.
Introduction
Historically, persons with hemophilia (PWH) were discouraged from participating in sports and strenuous physical exercise given the perceived risk of bleeding.1,2 It is therefore not surprising that early observations documented reduced proprioception, muscle strength, and physical endurance in PWH,3-5 along with a high prevalence of obesity.6-9 Obesity has specific health implications for PWH that include poor joint health, functional disability, increased factor utilization, and low quality-of-life scores.10-14 Recent studies, however, underscore a paradigm shift by documenting clear physical, medical, and psychosocial benefits of exercise and sports for PWH.15,16 Regular exercise and participation in appropriate sports is associated with improved endurance, muscle strength, and joint health.15 Exercise may also reduce the severity and frequency of bleeding,17,18 dampen the progression of preexisting joint damage, and improve social inclusion and adaptation.19,20
As the benefits of exercise and sports become apparent for PWH, treating physicians are faced with the dilemma of safely allowing sports participation while mitigating the potentially increased bleeding risk. In persons with severe or moderate hemophilia, prophylactic administration of factor concentrates before sports participation is recommended.21,22 Recently, use of intranasal (IN) desmopressin (Ferring Pharmaceuticals, Saint-Prex, Switzerland) before sports participation has been proposed in patients with mild hemophilia A (HA) to increase endogenous factor VIII activity (FVIII:C).16 IN desmopressin is expensive, requires fluid restriction, and may cause vomiting, headache, tachycardia, and seizures.23-26 In a recent study, we documented a 2.3-fold increase in FVIII:C in 8 adolescent males with mild-moderate HA with a standardized, moderate-intensity aerobic exercise regimen.27 This increase in FVIII:C with aerobic exercise may be protective against bleeding and may negate the need to administer IN desmopressin in adolescent males with mild HA before sports participation.
Here we report the principal results from a randomized trial of aerobic exercise vs IN desmopressin in adolescent males with mild HA. Our primary objective was to compare change in FVIII:C (measured as absolute- and fold-increase) arising from a standardized, moderate-intensity aerobic exercise regimen to that with IN desmopressin administration. Our hypothesis was that increase in FVIII:C with the exercise regimen would be noninferior to IN desmopressin. Our secondary objective was to investigate the impact of sequentially administered interventions (exercise and IN desmopressin) on FVIII:C. We hypothesized that exercise followed by IN desmopressin (or vice versa) would result in an additive increase in FVIII:C compared with either intervention alone.
Methods
Study cohort and protocol
The study (clinicaltrials.gov: #NCT03379974/#NCT03136003) was conducted simultaneously at the Hospital for Sick Children (SickKids), Ontario, Canada and Nationwide Children’s Hospital (NCH), Columbus, OH, USA. The NCH study cohort also included patients recruited from Cincinnati Children’s Hospital Medical Center (Cincinnati, OH) and Rainbow Babies and Children’s Hospital (Cleveland, OH), who traveled to Columbus for participation. The study was approved by the Institutional Review Board at all 4 centers. Adolescent/young adult males (age: 13-21 years) with mild HA (FVIII:C: 6%-50%) were solicited for participation. The mean of their 3 most recent FVIII:C was used to determine eligibility. Detailed exclusion criteria for participation can be found in supplemental Appendix 1, available on the Blood Web site.
Eligible subjects were approached during their annual clinic visits or via a recruitment brochure. Subjects who expressed an interest in participation were then contacted by a principal investigator (RK, US cohort; MC, Canadian cohort) to answer any questions and confirm an appointment for participation. Subjects were also contacted via telephone 1 week and 48 hours before the scheduled appointment to ensure that they continued to meet eligibility criteria. Upon arrival to the research center, written consent/assent was obtained from guardians/participants. Participants were evaluated by the principal investigators with a complete medical history and physical examination. Basal metabolic index (BMI) was determined, vital signs were collected, and the study protocol was explained to the participants.
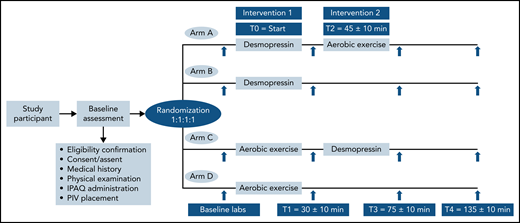
After consent was obtained, participants were randomized in 1:1:1:1, nonblinded allocation to 1 of 4 study arms: (1) arm A, IN desmopressin followed by exercise; (2) arm B, IN desmopressin alone; (3) arm C, exercise followed by IN desmopressin; (4) arm D, exercise alone (Figure 1). The randomization sequence was generated by a computer program and stratified by participating center. A peripheral IV (PIV) access was established in the forearm of all participants to facilitate blood sampling. Baseline blood work was obtained from the PIV, followed by the first intervention. The start of the first intervention was designated time zero (T0).
Participants randomized to arms A and B received IN desmopressin at T0 followed by rest for 30 ± 10 minutes before a second set of blood samples (T1; 30 ± 10 minutes from T0) were obtained. Participants in arm A started the graded exercise (T2), 45 ± 10 minutes from T0, whereas participants in arm B did not undergo any additional interventions. A similar schemata was followed for patients randomized to graded exercise first (arms C and D). They exercised on a cycle ergometer for approximately 10 to 15 minutes (T0), followed by rest for 15 ± 10 minutes before T1 blood specimens were obtained. Participants in arm C went on to receive IN desmopressin at T2, whereas participants in arm D did not receive any further interventions. Blood samples and vitals were repeated for all participants at 2 additional time points: T3 (75 ± 10 minutes from T0) and T4 (135 ± 10 minutes from T0). The study ended after T4 specimen collection. All participants received a modest compensation.
Sample size calculation
Sample size was estimated a priori for the primary objective. We anticipated a 2.5-fold increase in FVIII:C from baseline following IN desmopressin.28 Assuming a typical participant would begin with a baseline FVIII:C of 15%, we estimated that participants’ FVIII:C values would increase to an average of 37.5% following IN desmopressin (2.5-fold increase). We anticipated a similar FVIII:C increase with exercise. We rationalized that, if with exercise, FVIII:C increased to ≤22.5% (representing a 1.5-fold increase), this would constitute a clinically significant, inferior response compared with IN desmopressin. We thus estimated that 16 participants per group would provide 80% power to detect noninferiority between the exercise and IN desmopressin arms in terms of the mean FVIII:C, using a type I error rate of 2.5%, margin of noninferiority of −15.5%, and assuming a standard deviation in both groups of 15%.
Exercise protocol
All participants exercised on a stationary, calibrated cycle-ergometer (Upright Corivale, Lode, The Netherlands) using the previously validated, progressively incremental Godfrey protocol.27,29,30 Participants started cycling with an initial exercise load that was dependent on their height (supplemental Appendix 2). The workload was increased every minute in standard increments also based on the participant’s height. Participants continued to exercise until they reached 85% of their maximum predicted heart rate and were then maintained at that level of exertion for another 3-minutes. Heart rate (Polar HR monitor, Lachine, QC, Canada), blood pressure (Dash Monitor, GE Healthcare, Camarillo, CA), and oxygen saturation (forehead reflectance probe, Massimo Radical, Irvine, CA) were monitored throughout. Upon completion of planned exercise, the workload was decreased to zero watts, and participants were instructed to continue cycling at this cool-down rate for an additional 3 minutes before getting off the ergometer.
IN desmopressin
The dose of IN desmopressin administered was based on participant weight (150 mcg for participants < 50 kg; 300 mcg for participants ≥ 50 kg).28 The study team procured IN desmopressin through the research pharmacies at NCH and SickKids and provided the medication to participants randomized to arms A, B, and C.
Laboratory variables
Blood samples were collected at baseline, T1, T3, and T4 (Figure 1, supplemental Appendix 3). Complete blood count and platelet function analysis (PFA-100) were performed at the core hematology laboratories at NCH and SickKids for the US and Canadian cohorts, respectively. The following coagulation assays were performed at David Lillicrap’s research laboratory in Kingston, ON, Canada: (1) prothrombin time; (2) activated partial thromboplastin time; (3) 1-stage FVIII:C; (4) von Willebrand antigen (VWF:Ag); (5) von Willebrand factor platelet binding activity (VWF:GPIbM); (6) von Willebrand factor collagen binding (VWF:CBA); and (7) von Willebrand factor propeptide (VWF:pp). The methodologies for complete blood count and coagulation assays are well known and will not be described further.27 For tests performed in Kingston, platelet-poor plasma samples were prepared by centrifugation, flash frozen in liquid nitrogen, stored at −80 ± 10°C, and then batch shipped for testing.
Thrombin generation was assessed via calibrated automated thrombography (CAT), performed at Walter Kahr’s research laboratory at SickKids, Toronto, ON, Canada (for both US and Canadian cohorts). Platelet-poor plasma was obtained by centrifugation at >2500g from blood samples collected in tubes with 0.32% sodium citrate/1.45 μM corn trypsin inhibitor, consistent with established methods for assessing thrombin generation in clinical samples.31
Platelet-poor plasma samples were aliquoted, flash frozen in liquid nitrogen, stored at −80°C, and batch shipped to Toronto, ON, Canada (for US participants). Thrombin generation was assessed based on the methods developed by Hemker et al,32 which are optimal for studying thrombin generation in PWH. The results of the CAT assays were presented as a plot of thrombin concentration (nM) over time. Key parameters studied included peak thrombin generation (PTG, nmol/L) and endogenous thrombin potential (ETP; nmol/L × min, calculated as area under the thrombogram curve).
Statistical analysis
Standard statistical methods were used to summarize the parameters: frequency and percent for categorical parameters; and mean (±standard deviation [SD]) and median and interquartile range (IQR) for ordinal parameters. Mean (±SD) was used for normally distributed data, whereas use of median (IQR) was reserved for non-normally distributed data. To test for noninferiority of aerobic exercise compared with IN desmopressin with regard to FVIII:C increase, a 1-sided, 2-sample t test was calculated. To compare pre- and postintervention levels of the other laboratory assays (T1/T0), we performed a paired t test when the paired differences were normally distributed; a nonparametric sign test was performed in cases where the paired differences were non-normally distributed. To compare the impact of sequentially administered interventions on laboratory assays, we used a linear mixed-effects model with a random intercept for each participant. Pairwise analyses were adjusted for multiple comparisons using the Tukey method. Statistical analyses were completed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Clinical characteristics of study cohort
Thirty-two participants were enrolled: 17 at SickKids and 15 at NCH. Mean age (±SD) at study participation was 16.1 (±2.6) years, and mean baseline FVIII:C (on day of participation) was 27.9% (±18.4%). Mean historical FVIII:C for the study cohort was 19.5% (±11.6%) and was significantly lower than mean baseline FVIII:C on day of participation (P < .0001). Thirty of 32 participants had previously undergone genetic testing, and 28 were found to have putative disease-causative variants in their F8 gene (27 missense variants and 1 splice site variant in exon 11 with a baseline FVIII:C of 8%). For the 4 participants who had either not undergone genetic testing or in whom a disease-causative variant was not identified, type 2N von Willebrand disease and combined factor V/FVIII deficiency were excluded by VWF gene sequencing and checking the factor V assay, respectively.33 Baseline BMI for the entire cohort was 22.9 (±4.6) kg/m2. Twenty-eight participants had previously undergone a desmopressin challenge, 20 of whom were identified as “responders” and 5 as “partial responders” by their primary hematologists. One participant was labeled as a nonresponder, and the response was unknown for 2 participants. Baseline demographic characteristics were similar in participants randomized to the IN desmopressin first arms (arms A and B), and those randomized to the exercise first arms (arms C and D; Table 1).
Baseline demographics in patients randomized to exercise vs desmopressin
| . | Desmopressin first (arms A and B) . | Aerobic exercise first (arms C and D) . |
|---|---|---|
| Subjects, n (%) | 16 | 16 |
| NCH | 8 (50) | 7 (44) |
| SickKids | 8 (50) | 9 (56) |
| Mean age at study visit, y (±SD) | 15 (±2) | 17 (±3) |
| Mean historical FVIII:C, % (±SD) | 17 (±9) | 23 (±14) |
| Mean BMI, kg/m2 (±SD) | 22 (±5) | 24 (±4) |
| Previous desmopressin challenge, n (%) | 15 (94) | 13 (81) |
| Desmopressin challenge response, n (%) | ||
| Responder | 10 (67) | 10 (77) |
| Partial responder | 3 (20) | 2 (15) |
| Nonresponder | 1 (7) | 0 |
| Unknown | 1 (7) | 1 (8) |
| Previous desmopressin use, n (%) | 9 (56) | 9 (56) |
| . | Desmopressin first (arms A and B) . | Aerobic exercise first (arms C and D) . |
|---|---|---|
| Subjects, n (%) | 16 | 16 |
| NCH | 8 (50) | 7 (44) |
| SickKids | 8 (50) | 9 (56) |
| Mean age at study visit, y (±SD) | 15 (±2) | 17 (±3) |
| Mean historical FVIII:C, % (±SD) | 17 (±9) | 23 (±14) |
| Mean BMI, kg/m2 (±SD) | 22 (±5) | 24 (±4) |
| Previous desmopressin challenge, n (%) | 15 (94) | 13 (81) |
| Desmopressin challenge response, n (%) | ||
| Responder | 10 (67) | 10 (77) |
| Partial responder | 3 (20) | 2 (15) |
| Nonresponder | 1 (7) | 0 |
| Unknown | 1 (7) | 1 (8) |
| Previous desmopressin use, n (%) | 9 (56) | 9 (56) |
Data available for 31 of 32 subjects.
For participants randomized to 1 of the 3 exercise arms (n = 22), mean duration (±SD) to achieve 85% of maximum predicted heart rate was 9.5 (±1.8) minutes, and mean duration of exercise was 12.9 (±2.4) minutes. The study protocol was well tolerated; 1 participant reported transient headache, and 1 reported palpitation after the exercise protocol. Similarly, 1 participant reported headache and 1 reported chest pain after IN desmopressin. All 4 participants had stable vital signs during these mild transient adverse effects, and none required pharmacologic intervention. No participant had to prematurely discontinue the study protocol because of an adverse event.
Change in FVIII:C with exercise vs desmopressin
For patients randomized to IN desmopressin first arms (arms A and B), the mean (±SD) FVIII:C increased from 24.5% (±13.5%) at baseline to 45.7% (±24%) at T1, denoting a 1.9-fold increase. For participants randomized to aerobic exercise upfront (arms C and D), the mean FVIII:C increased from 31.4% (±22.7%) at baseline to 54.7% (±49.8%) at T1, denoting a 1.7-fold increase. The immediate increase in FVIII:C with exercise was similar to that with IN desmopressin (noninferiority P = .04; Figure 2A), although this did not meet our a priori significance level for noninferiority (P < .025).
Immediate change in hemostatic variables (T1 vs baseline) with IN desmopressin compared with exercise. (A) FVIII:C (%). (B) VWF:Ag (%). (C) VWF:GpIbM (%). (D) Platelet count. (E) Collage/epinephrine closure time (s). (F) Collagen/ADP closure time (s).
Immediate change in hemostatic variables (T1 vs baseline) with IN desmopressin compared with exercise. (A) FVIII:C (%). (B) VWF:Ag (%). (C) VWF:GpIbM (%). (D) Platelet count. (E) Collage/epinephrine closure time (s). (F) Collagen/ADP closure time (s).
Change in hemostatic indices with exercise vs desmopressin
Changes in VWF assays were statistically similar between the 2 study arms: VWF:Ag (1.3-fold increase with IN desmopressin compared with 1.2-fold increase with exercise; P = .36; Figure 2B); VWF:GPIbM (1.4-fold increase with IN desmopressin compared with 1.3-fold increase with exercise; P = .09; Figure 2C); VWF:CBA (1.3-fold with IN desmopressin compared with 1.2-fold with exercise; P = .23; data not shown); and VWF:pp (3-fold increase with IN desmopressin compared with 2-fold increased with exercise; P = .11; data not shown). There was no change in the platelet count with IN desmopressin compared with a 1.1-fold (T1 vs baseline) increase in platelet count with exercise (P = .0004; Figure 2D). IN desmopressin was associated with a 0.6-fold decrease in the collagen/epinephrine (Col/Epi) closure time, whereas exercise was not associated with a change in Col/Epi closure time (P = .007; Figure 2E). Fold decrease in collagen/adenosine diphosphate (Col/ADP) closure times was similar in both arms (0.9-fold with IN desmopressin and 0.7-fold with exercise; P = .57; Figure 2F). Hematocrit remained relatively stable in both arms (data not shown).
Longitudinal change in hemostatic indices over the course of the study
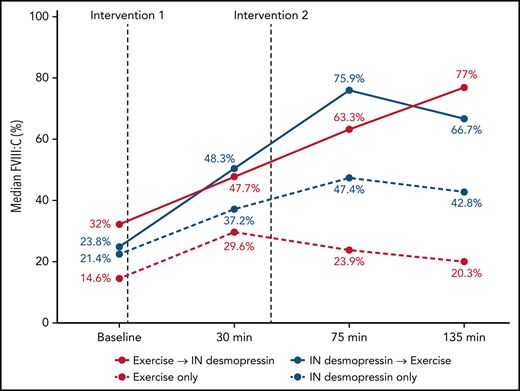
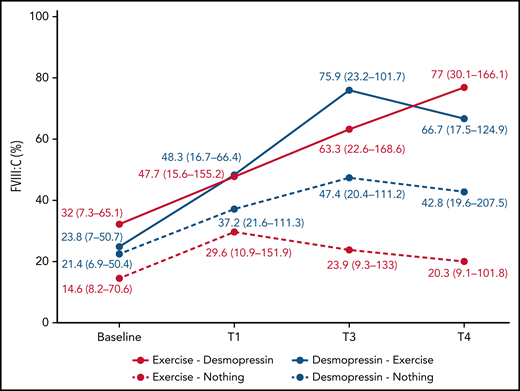
In participants randomized to IN desmopressin alone (arm B), the highest median FVIII:C was noted at T3 (2.2-fold compared with baseline) and plateaued by T4 (2-fold compared with baseline; Table 2). In contrast, in participants randomized to exercise alone (arm D), highest median FVIII:C was noted at T1 (2-fold compared with baseline) but began declining by T3 (1.6-fold compared with baseline; Figure 3). In participants randomized to IN desmopressin + exercise (arm A), the highest median FVIII:C was noted at T3 (3.2-fold compared with baseline). Participants randomized to exercise + IN desmopressin (arm C) demonstrated progressively increasing FVIII:C, and the highest median FVIII:C was noted at T4 (2.4-fold compared with baseline). Although there were no statistically significant differences in median FVIII:C at any time point between the 4 regimens, it is interesting to note that 67% (10 of 15) of participants randomized to arms A and C (combined IN desmopressin + exercise) had a normal (ie, >50%) FVIII:C at T3, and 60% (9 of 15) continued to have a normal FVIII:C at T4. In contrast, only 35% (6 of 17) and 24% (4 of 17) of participants randomized to arms B and D (IN desmopressin or exercise monotherapy) had normal FVIII:C at T3 and T4, respectively.
Changes in hemostatic parameters over the course of the study
| . | Study arm . | Baseline; median (IQR) . | T1; median (IQR) . | Fold change (vs baseline) . | T3; median (IQR) . | Fold change (vs baseline) . | T4; median (IQR) . | Fold change (vs baseline) . |
|---|---|---|---|---|---|---|---|---|
| FVIII:C (%) | Arm A | 23.8 (7-50.7) | 48.3 (16.7-66.4) | 2 | 75.9 (23.2-101.7) | 3.2 | 66.7 (17.5-124.9) | 2.8 |
| Arm B | 21.4 (6.9-50.4) | 37.2 (21.6-111.3) | 1.7 | 47.4 (20.4-111.2) | 2.2 | 42.8 (19.6-207.5) | 2 | |
| Arm C | 32 (7.3-65.1) | 47.7 (15.6-155.2) | 1.5 | 63.3 (22.6-168.6) | 2 | 77 (30.1-166.1) | 2.4 | |
| Arm D | 14.6 (8.2-70.6) | 29.6 (10.9-151.9) | 2 | 23.9 (9.3-133) | 1.4 | 20.3 (9.1-101.8) | 1.4 | |
| VWG:Ag (%) | Arm A | 84.9 (80.4-121.3) | 118.8 (107.3-176) | 1.4 | 173.9 (145.2-266.8) | 2*† | 200.8 (128.2-266.8) | 2.4*† |
| Arm B | 131.5 (89-172) | 156.6 (117.3-197.7) | 1.2 | 181.6 (139.6-227.1) | 1.4*† | 265.6 (145.2-288.9) | 2*† | |
| Arm C | 88.7 (64.7-123.9) | 101.4 (71.1-112.5) | 1.1 | 107.5 (89.1-119.5) | 1.2 | 97.3 (93.8-165.5) | 1.1 | |
| Arm D | 78.8 (61.2-89) | 80.3 (65.6-134.7) | 1 | 72.8 (56.4-130) | 1 | 76.8 (57.4-125.3) | 1 | |
| VWF:Gp1bM (%) | Arm A | 88.7 (68.2-126.9) | 145.7 (114.9-169.3) | 1.6 | 207.4 (169.7-286.8) | 2.3* | 221.8 (233.8-152.1) | 2.5* |
| Arm B | 139.7 (77.3-157.7) | 179.3 (145.9-210.6) | 1.3 | 192.8 (99-251.2) | 1.4* | 185.7 (136.4-228.6) | 1.3* | |
| Arm C | 77.9 (56.3-127.2) | 113.5 (82.8-141.8) | 1.5 | 141.3 (91.7-156.1) | 1.8 | 146.9 (119.1-214.7) | 1.9* | |
| Arm D | 75.9 (58.3-94.9) | 76.2 (67.4-138.7) | 1 | 73.6 (63.4-137) | 1 | 72.3 (63.9-124.1) | 1 | |
| Platelet count (x103/L) | Arm A | 233.5 (212-275) | 236.5 (203.5-289) | 1 | 297 (234-326.5) | 1.3 | 226.5 (195-260.8) | 1 |
| Arm B | 282 (175.3-298.3) | 263 (171-302.8) | 0.9 | 265.5 (165.8-293) | 0.9 | 256.5 (167.3-296.3) | 0.9 | |
| Arm C | 235 (200-253) | 246 (219-275) | 1 | 216 (199-267) | 0.9 | 222 (200-241) | 0.9 | |
| Arm D | 260 (255.5-292.5) | 281 (245.5-332) | 1.1 | 235 (216-274.5) | 0.9 | 264 (223.5-286) | 1 | |
| Col/Epi closure time (s) | Arm A | 143.5 (122.3-153.5) | 82 (75-89) | 0.6 | 77.5 (70.8-83.8) | 0.5* | 99.5 (75.3-110) | 0.7* |
| Arm B | 139 (111.3-148.3) | 78.5 (74.3-111.3) | 0.6 | 91 (71.5-100.5) | 0.7* | 94.5 (77.5-117.3) | 0.7* | |
| Arm C | 129.5 (97.8-149.8) | 121 (106-173) | 0.9 | 102 (76-119) | 0.8* | 96 (82-112) | 0.7* | |
| Arm D | 142 (128.5-177.5) | 143 (113-162.5) | 1 | 150 (113-177.5) | 1 | 166 (110-183) | 1.1 | |
| Col/ADP closure time (s) | Arm A | 100.5 (74.3-119.5) | 77 (60-81) | 0.8 | 58 (53-76.3) | 0.6* | 76.5 (65.5-87.3) | 0.8* |
| Arm B | 80.5 (61.5-100.8) | 64.5 (48.8-81) | 0.8 | 63 (59.3-66) | 0.8* | 62 (56-69.8) | 0.8* | |
| Arm C | 104 (85-115.5) | 83 (62-97) | 0.8 | 75 (65-78) | 0.7* | 67 (64-79) | 0.6* | |
| Arm D | 105 (90-120.5) | 103 (79.5-108.5) | 1 | 91 (85-113.5) | 0.9 | 103 (91.5-130) | 1 | |
| Hematocrit (%) | Arm A | 41.7 (40.6-44.6) | 41 (37.8-42.3) | 1 | 41.6 (40.3-44.9) | 1 | 40.2 (36.7-40.6) | 1 |
| Arm B | 43.6 (42.3-45.6) | 41.4 (40-41.4) | 0.9 | 40.5 (39.6-43.1) | 0.9 | 40.4 (39.4-42.7) | 0.9 | |
| Arm C | 44.2 (39.8-45.4) | 44.9 (41.45.9) | 1 | 40.9 (37.7-42.6) | 0.9 | 41.4 (36.8-42.7) | 0.9 | |
| Arm D | 43.3 (41.3-47.7) | 44.5 (42.6-46.6) | 1 | 40.4 (39.2-43.2) | 0.9 | 40.6 (39.3-44.5) | 0.9 |
| . | Study arm . | Baseline; median (IQR) . | T1; median (IQR) . | Fold change (vs baseline) . | T3; median (IQR) . | Fold change (vs baseline) . | T4; median (IQR) . | Fold change (vs baseline) . |
|---|---|---|---|---|---|---|---|---|
| FVIII:C (%) | Arm A | 23.8 (7-50.7) | 48.3 (16.7-66.4) | 2 | 75.9 (23.2-101.7) | 3.2 | 66.7 (17.5-124.9) | 2.8 |
| Arm B | 21.4 (6.9-50.4) | 37.2 (21.6-111.3) | 1.7 | 47.4 (20.4-111.2) | 2.2 | 42.8 (19.6-207.5) | 2 | |
| Arm C | 32 (7.3-65.1) | 47.7 (15.6-155.2) | 1.5 | 63.3 (22.6-168.6) | 2 | 77 (30.1-166.1) | 2.4 | |
| Arm D | 14.6 (8.2-70.6) | 29.6 (10.9-151.9) | 2 | 23.9 (9.3-133) | 1.4 | 20.3 (9.1-101.8) | 1.4 | |
| VWG:Ag (%) | Arm A | 84.9 (80.4-121.3) | 118.8 (107.3-176) | 1.4 | 173.9 (145.2-266.8) | 2*† | 200.8 (128.2-266.8) | 2.4*† |
| Arm B | 131.5 (89-172) | 156.6 (117.3-197.7) | 1.2 | 181.6 (139.6-227.1) | 1.4*† | 265.6 (145.2-288.9) | 2*† | |
| Arm C | 88.7 (64.7-123.9) | 101.4 (71.1-112.5) | 1.1 | 107.5 (89.1-119.5) | 1.2 | 97.3 (93.8-165.5) | 1.1 | |
| Arm D | 78.8 (61.2-89) | 80.3 (65.6-134.7) | 1 | 72.8 (56.4-130) | 1 | 76.8 (57.4-125.3) | 1 | |
| VWF:Gp1bM (%) | Arm A | 88.7 (68.2-126.9) | 145.7 (114.9-169.3) | 1.6 | 207.4 (169.7-286.8) | 2.3* | 221.8 (233.8-152.1) | 2.5* |
| Arm B | 139.7 (77.3-157.7) | 179.3 (145.9-210.6) | 1.3 | 192.8 (99-251.2) | 1.4* | 185.7 (136.4-228.6) | 1.3* | |
| Arm C | 77.9 (56.3-127.2) | 113.5 (82.8-141.8) | 1.5 | 141.3 (91.7-156.1) | 1.8 | 146.9 (119.1-214.7) | 1.9* | |
| Arm D | 75.9 (58.3-94.9) | 76.2 (67.4-138.7) | 1 | 73.6 (63.4-137) | 1 | 72.3 (63.9-124.1) | 1 | |
| Platelet count (x103/L) | Arm A | 233.5 (212-275) | 236.5 (203.5-289) | 1 | 297 (234-326.5) | 1.3 | 226.5 (195-260.8) | 1 |
| Arm B | 282 (175.3-298.3) | 263 (171-302.8) | 0.9 | 265.5 (165.8-293) | 0.9 | 256.5 (167.3-296.3) | 0.9 | |
| Arm C | 235 (200-253) | 246 (219-275) | 1 | 216 (199-267) | 0.9 | 222 (200-241) | 0.9 | |
| Arm D | 260 (255.5-292.5) | 281 (245.5-332) | 1.1 | 235 (216-274.5) | 0.9 | 264 (223.5-286) | 1 | |
| Col/Epi closure time (s) | Arm A | 143.5 (122.3-153.5) | 82 (75-89) | 0.6 | 77.5 (70.8-83.8) | 0.5* | 99.5 (75.3-110) | 0.7* |
| Arm B | 139 (111.3-148.3) | 78.5 (74.3-111.3) | 0.6 | 91 (71.5-100.5) | 0.7* | 94.5 (77.5-117.3) | 0.7* | |
| Arm C | 129.5 (97.8-149.8) | 121 (106-173) | 0.9 | 102 (76-119) | 0.8* | 96 (82-112) | 0.7* | |
| Arm D | 142 (128.5-177.5) | 143 (113-162.5) | 1 | 150 (113-177.5) | 1 | 166 (110-183) | 1.1 | |
| Col/ADP closure time (s) | Arm A | 100.5 (74.3-119.5) | 77 (60-81) | 0.8 | 58 (53-76.3) | 0.6* | 76.5 (65.5-87.3) | 0.8* |
| Arm B | 80.5 (61.5-100.8) | 64.5 (48.8-81) | 0.8 | 63 (59.3-66) | 0.8* | 62 (56-69.8) | 0.8* | |
| Arm C | 104 (85-115.5) | 83 (62-97) | 0.8 | 75 (65-78) | 0.7* | 67 (64-79) | 0.6* | |
| Arm D | 105 (90-120.5) | 103 (79.5-108.5) | 1 | 91 (85-113.5) | 0.9 | 103 (91.5-130) | 1 | |
| Hematocrit (%) | Arm A | 41.7 (40.6-44.6) | 41 (37.8-42.3) | 1 | 41.6 (40.3-44.9) | 1 | 40.2 (36.7-40.6) | 1 |
| Arm B | 43.6 (42.3-45.6) | 41.4 (40-41.4) | 0.9 | 40.5 (39.6-43.1) | 0.9 | 40.4 (39.4-42.7) | 0.9 | |
| Arm C | 44.2 (39.8-45.4) | 44.9 (41.45.9) | 1 | 40.9 (37.7-42.6) | 0.9 | 41.4 (36.8-42.7) | 0.9 | |
| Arm D | 43.3 (41.3-47.7) | 44.5 (42.6-46.6) | 1 | 40.4 (39.2-43.2) | 0.9 | 40.6 (39.3-44.5) | 0.9 |
P < 0.05 (compared with arm D).
P < 0.05 (compared with arm C).
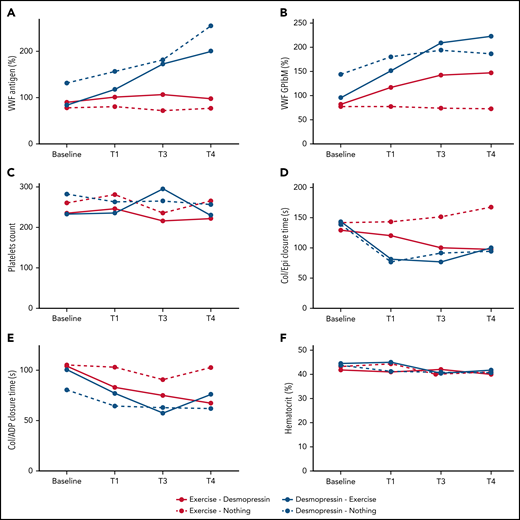
Participants randomized to IN desmopressin first (arms A and B) had significantly higher median VWF:Ag compared with participants randomized to exercise alone (arm D) by T3 (P < .0001 and P = .001, respectively) and T4 (P < .0001 for both). They also had significantly higher median VWF:Ag at both T3 and T4 compared with participants randomized to exercise + IN desmopressin (arm C) (P values: 0.005 and 0.05, respectively, for T3 vs baseline; P values: 0.03 and 0.002, respectively, for T4 vs baseline; Figure 4A). There was only moderate correlation between VWF:Ag and FVIII:C at various time points (ρ: 0.34-0.58; supplemental Appendix 4).
Change in median hemostatic variables across the 4 study arms. (A) VWF:Ag (%). (B) VWF:GPIbM (%). (C) Platelet count. (D) Collage/epinephrine closure time (s). (E) Collagen/ADP closure time (s). (F) Hematocrit (%).
Change in median hemostatic variables across the 4 study arms. (A) VWF:Ag (%). (B) VWF:GPIbM (%). (C) Platelet count. (D) Collage/epinephrine closure time (s). (E) Collagen/ADP closure time (s). (F) Hematocrit (%).
With regard to VWF activity, participants randomized to IN desmopressin first (arms A and B) had a significantly higher median VWF:GP1bM activity compared with participants randomized to exercise alone (arm D) by T3 (P < .0001 and .002, respectively). By T4, all IN desmopressin-containing arms (arms A, B, and C) had a significantly higher median VWF:GPIbM activity compared with arm D (P < .0001, .0006, and .05, respectively; Figure 4B). There were no statistically significant differences in platelet counts at any of the time points between the 4 arms, although all patients undergoing aerobic exercise experienced a brief rise in their platelet counts with exercise (Figure 4C).
Changes in PFA-100 closure times paralleled changes in VWF activity (Figure 4D-E). Participants randomized to IN desmopressin-containing arms (arms A, B, and C) had significantly shorter Col/Epi closure times compared with participants randomized to exercise only (arm D) at both T3 (P < .0001, .0001, and .005, respectively) and T4 (P = .005, .007, and .003, respectively). Similarly, participants randomized to arms A, B, and C had significantly shorter Col/ADP closure times compared with participants randomized to arm D at both T3 (P < .0001, .0001, and .008, respectively) and T4 (P < .002, .0001, and .0007, respectively). There were no significant changes in hematocrit across the 4 study arms (Figure 4F).
CAT results
In participants randomized to an IN desmopressin first arm (arms A and B), the mean PTG increased from 18.4 (±8.8) nmol/L to 29.3 (±16.1) nmol/L, whereas in participants randomized to aerobic exercise upfront (arms C and D), mean PTG increased from 21.9 (±14.5) nmol/L to 30.7 (±22.5) nmol/L (P = .56). Similarly, the change in mean ETP was not significantly different between the 2 arms: 281.3 (±124.8) to 407.3 (±182.3) nmol/L × min with IN desmopressin vs 328.2 (±197.2) to 396.5 (± 232.8) nmol/L × min with aerobic exercise (P = .22). Changes in PTG and ETP across the 4 study arms are elaborated in supplemental Appendix 5.
Subgroup analysis of Canadian and US cohorts
On 21 July 2020, a voluntary recall of IN desmopressin was issued by Ferring Pharmaceuticals because of concerns of “superpotency.”34 This recall was issued after routine testing showed out-of-specification assay results in some vials of IN desmopressin. An internal review confirmed that all US study participants had received the impacted product, whereas none of the Canadian participants had been exposed to the superpotent medication. A subgroup analysis was performed, comparing the absolute- and fold-increase in FVIII:C between the Canadian and US cohorts randomized to an IN desmopressin first arm (arms A and B; T1 vs baseline). Median FVIII:C in the Canadian cohort increased from 26.7% to 33.6%, denoting a 1.3-fold increase. In comparison, median FVIII:C in the US cohort increased from 15.6% to 33.9%, denoting a 2.2-fold increase (P = .06). Of note, none of the participants exposed to IN desmopressin experienced severe adverse effects, and all affected subjects were notified of the recall.
Discussion
In this randomized study, we documented that approximately 12 minutes of moderate-intensity aerobic exercise was associated with a similar immediate (30-minute) increase in FVIII:C and VWF activity compared with IN desmopressin. However, the prohemostatic response associated with exercise was short lived, with near complete reversal by approximately 2 hours. In contrast, participants randomized to receive IN desmopressin (arm B) had sustained improvements in their hemostatic parameters. In particular, their median VWF:Ag, VWF:GPIbM, and PFA-100 closure times were significantly better at 75 and 135 minutes compared with participants randomized to the exercise alone (arm D).
The overarching goal of our study was to investigate whether adolescents with mild HA would derive a similar hemostatic benefit from about 12 minutes of moderate-intensity “warm-up” exercise compared with IN desmopressin before participating in potentially injury-provoking sports. Our findings indicate that IN desmopressin results in a more sustained improvement in hemostasis, whereas moderate-intensity “warm-up” alone leads to a short burst of hemostatic improvement followed by a steady decline. As such, a moderate-intensity warm up regimen may not provide adequate hemostatic protection before organized sports activities that typically last for 90 to 180 minutes. Physical activity in younger adolescents, particularly nonorganized sports as occurs in school playgrounds, is typically characterized by short bursts of high-intensity exercise interrupted by periods of rest.30 Adolescents with mild HA likely derive hemostatic benefit from these short periods of high-intensity exercise, although our current study does not investigate the hemostatic impact of repeated exercise bursts on FVIII:C. Although a majority (≥60%) of patients randomized to combination of exercise and IN desmopressin (arms A and C) had >50% FVIII:C at 75 and 135 minutes, there was significant interpatient variability that prevents us from making clinical recommendations at this time. We are currently investigating clinical variables including BMI and baseline physical activity that may predict which patients will derive greater hemostatic benefit from exercise alone or in combination with IN desmopressin.
This study builds on previous work from our group and others documenting the hemostatic benefit of exercise in PWH. In 1984, Koch35 documented a 1.2-fold increase in FVIII:C in 4 boys with mild HA after a cycle ergometer based exercise protocol. In 2006, Roya et al36 studied 10 patients with mild-moderate HA (mean age: 24.5 years) and documented a 1.3-fold increase in FVIII:C with 23 to 46 minutes of cycle ergometer–based exercise. More recently, in 2013, Groen et al37 had 15 patients with mild-moderate HA (mean age: 26.5 years) perform intense stationary cycling until exhaustion. Blood work was done before and 8 minutes after exercise, and the authors noted a 2.5-fold increase in FVIII:C in addition to significant increases in both VWF:Ag and VWF:RCo.
We recently published a pilot study that investigated the impact of a cycle ergometer–based exercise protocol on hemostasis in children with hemophilia.27 Thirty children with hemophilia (both hemophilia A and hemophilia B, all severities) were enrolled. Although we documented a significant improvement in multiple hemostatic parameters, the effect was particularly pronounced in 8 adolescent males with mild-moderate HA. In this cohort, we noted a 2.3-fold increase in FVIII:C immediately after exercise, which remained significantly elevated at 1.9-fold 1 hour after completion of exercise. These observations prompted us to compare the hemostatic improvements associated with exercise to IN desmopressin that historically has been associated with a two- to fourfold increase in FVIII:C and VWF in patients with mild-moderate HA.28,38,39
Desmopressin causes the release of endogenous VWF from endothelial cell Weibel-Palade bodies through a G-protein–coupled arginine vasopressin receptor 2 signaling mechanism.40-42 Desmopressin additionally increases platelet adhesiveness and shortens bleeding time independent of its effects on FVIII and VWF.43,44 Postexercise FVIII:C and VWF increment is also hypothesized to involve release of Weibel-Palade body contents (via β adrenergic receptor signaling).45,46 Given the significantly short-lived impact of exercise, we hypothesize that details of the release of presynthesized VWF from Weibel-Palade bodies is different for the 2 exocytosis agonists. With respect to the differential effect of exercise vs desmopressin in raising platelet counts, we hypothesize that during aerobic exercise, platelets are transiently released from splenic stores.
Limitations of this study include a sample size that was only powered to investigate our primary objective. It remains plausible that the sequential administration of exercise and IN desmopressin is associated with an additive increase in FVIII:C, although this did not meet statistical significance in the current investigation. An additional limitation is that participation in the current randomized trial was restricted to boys, although mild HA can also affect females.47 Of note, adolescent females were enrolled on a nonrandomized prospective study to investigate the impact of exercise on hemostasis (clinicaltrials.gov: #NCT03379974), and their results will be reported separately. Last, study participants were asked to abstain from exercise for 72 hours before participation to ensure that VWF and FVIII stores were at maximum. It remains plausible that in subjects who exercise regularly, the changes in hemostatic indices would be less substantial with both exercise and IN desmopressin. The biggest limitation of the study, however, was the fact that participants enrolled at NCH were exposed to “superpotent” desmopressin. The subgroup analysis shows that the Canadian participants had a 1.3-fold increase in FVIII:C with IN desmopressin compared with a 2.4-fold increase in the US cohort. Although not statistically significant (P = .06), we believe this difference may be clinically relevant but was beyond our control. It is reassuring that despite ∼50% of the cohort being exposed to supratherapeutic drug doses, the immediate increase in FVIII:C with exercise was similar to IN desmopressin.
In summary, this study confirms our initial observation that moderate-intensity aerobic exercise is associated with a nearly twofold immediate increase in FVIII:C activity in teenage boys with mild HA. The increase in FVIII:C with exercise was similar to the IN desmopressin response. However, exercise-mediated hemostatic changes were short lived, with near complete reversal to baseline by 2 hours. In contrast, hemostatic changes associated with IN desmopressin were more sustained. Our work adds to a growing body of literature documenting the hemostatic advantages of exercise in patients with bleeding disorders. We are currently investigating females with mild HA and hope to investigate the impact of repeated bursts of exercise on hemostatic markers of coagulation.
Acknowledgments
R.K. received the 2016 Hemostasis and Thrombosis Mentored Research award (HTRS MRA) supported by an educational grant by Bioverativ Therapeutics to fund the US arm of the study. M.C. received the 2016 Canadian Hemophilia Society (Care Until Cure program) award to fund the Canadian arm of the study. A.G. is a recipient of the 2016 National Hemophilia Foundation Physical Therapy Excellence fellowship.
Authorship
Contribution: R.K., A.L.D., and M.C. designed the study, recruited patients, conducted the study, collected data, analyzed data, and wrote the manuscript; J.E.S., A.G., V.B., and P.W. recruited patients, conducted the study, collected data, and critically reviewed/edited the manuscript; W.H.A.K., J.S., F.G.P., A.W., B.A.K., M.L.R., and D.L. analyzed data and critically reviewed/edited the manuscript; and C.T. and S.A. recruited patients and critically reviewed/edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manuel Carcao, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8, Canada; e-mail: manuel.carcao@sickkids.ca; and Riten Kumar, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, 300 Longwood Ave, Boston, MA 02115; e-mail: riten.kumar@childrens.harvard.edu.
For original data, please contact riten.kumar@childrens.harvard.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal