Abstract
HIV infection increases cancer risk and is linked to cancers associated to infectious agents classified as carcinogenic to humans by the International Agency for Research on Cancer. Lymphomas represent one of the most frequent malignancies among individuals infected by HIV. Diffuse large B-cell lymphoma remains a leading cancer after the introduction of combined antiretroviral therapy (cART). The incidence of other lymphomas including Burkitt lymphoma, primary effusion lymphomas, and plasmablastic lymphoma of the oral cavity remain stable, whereas the incidence of Hodgkin lymphoma and Kaposi sarcoma-associated herpesvirus (KSHV)-associated multicentric Castleman disease has increased. The heterogeneity of lymphomas in individuals infected by HIV likely depends on the complexity of involved pathogenetic mechanisms (ie, HIV-induced immunosuppression, genetic abnormalities, cytokine dysregulation, and coinfection with the gammaherpesviruses Epstein-Barr virus and KSHV) and the dysregulation of the immune responses controlling these viruses. In the modern cART era, standard treatments for HIV-associated lymphoma including stem cell transplantation in relapsed/refractory disease mirror that of the general population. The combination of cART and antineoplastic treatments has resulted in remarkable prolongation of long-term survival. However, oncolytic and immunotherapic strategies and therapies targeting specific viral oncogenes will need to be developed.
Introduction
Lymphomas are among the most common malignancies among people living with HIV.1 Since the early stages of the AIDS pandemic, HIV infection has had a strong impact on cancer incidence. The original case definition of AIDS included the association of HIV infection with Kaposi sarcoma (KS) and primary central nervous system lymphoma (PCNSL).2 Subsequent epidemiologic studies demonstrated that HIV infection increases the risk of other cancers.3 Before the development of effective combination antiretroviral therapy (cART), the relative risk for non-Hodgkin lymphoma (NHL) was estimated as 60- to 200-fold compared with the general population.4-6 Consistent with epidemiologic data, HIV was classified as carcinogenic to humans by the International Agency for Research on Cancer.7 HIV plays a carcinogenic and lymphomagenic role through immunosuppression, immune dysregulation, chronic antigenic stimulation, and possibly direct viral activity via the secretion of viral proteins.7-10
Starting in 1992, AIDS-defining cancers included, in addition to PCNSL, Burkitt lymphoma (BL), diffuse large B-cell lymphomas (DLBCL), and invasive cervical cancer.11,12 The definition of AIDS-defining lymphomas has not officially been revised, but now it refers to all aggressive B-cell NHL in HIV-infected patients, including new subtypes observed more frequently in HIV-infected individuals than in noninfected individuals, such as primary effusion lymphoma (PEL),13 large B-cell lymphoma arising in multicentric Castleman disease (MCD),14 and plasmablastic lymphoma (PBL) of the oral cavity type.15 Then, the categories of hematologic cancers in individuals infected by HIV include the same aggressive lymphomas that develop sporadically in the absence of HIV infection and lymphomas occurring specifically in patients with AIDS,16 despite cART use.17,18 The incidence of all AIDS-defining malignancies has significantly decreased in the cART era, but the risk remains significantly elevated for each AIDS-defining NHL subtype, specifically DLBCL standardized incidence ratio (SIR 10), and BL (SIR 20).19-23 It is noteworthy that among cART users, the risk for Hodgkin lymphoma (HL) has become comparable to that for NHL (SIR 8-20).19,20,24
The widespread availability of cART, along with better management of opportunistic infections, led to a substantial improvement in life expectancy for individuals infected by HIV. This has resulted in changes in the demographics of population of individuals infected by HIV who are now older and generally have higher CD4 cell counts.25 The combination of cART and antineoplastic treatment has produced prolonged survival in individuals with HIV infection and cancer. However, lymphoma survival still differs between HIV-infected patients and the general population, which emphasizes the need to wider dissemination of the best treatment protocol. In particular, with the introduction of cART in clinical practice, it is possible to consider dose-intensive chemotherapy regimens plus cART, including autologous and even allogenic stem cell transplantation in patients with relapsed/refractory lymphoma. This review includes an update on the spectrum of hematologic cancers in individual infected by HIV, on the main viral and molecular pathogenetic pathways, and on the management of these patients in the modern cART era.
Distribution over time of lymphoma histotypes
Table 1 shows data from 2 studies on large cohort based on lymphoma patients from the United States and Europe, respectively.25,26 In the late-cART era (2006-2015) the most common histotypes of lymphoma are DLBCL (Figure 1), shifting from 63% in the pre-cART era to 35% to 37% in the late-cART era, and BL (Figure 2), shifting from 3% in the pre-cART era (1986-1995) to 16% to 20% in the late-cART era. HL, 1 of the most frequent categories in the non–AIDS-defining cancers,20 increased from 20% to 26%. Therefore, over 3 decades, there has been an increase in lymphoma entities (ie, BL and HL), which are associated with moderate immunosuppression but adverse outcome.25,26 Conversely, in the late cART decade, aggressive types of lymphomas (ie, PBL and PEL) have been observed more frequently than the previous decades (Table 1). Another study on a cohort based on patients with lymphoma from Japan27 analyzed the histologic differences between cases in the pre-cART era (1987-1997) and those in the cART era (1997-2012). Putting the results of the 3 studies together, the incidence of BL has increased significantly in the United States, Europe, and Japan.25-27 The incidence of DLBCL has significantly decreased in Europe and Japan, but it is stable in the United States.25-27 In the highly active antiretroviral therapy era, DLBCL of the germinal center B-cell subtype is more common than previously seen, whereas DLBCL of the activated B-cell (immunoblastic subtype) has decreased.28 The incidence of HL was very low in the pre-cART era27 and was probably underestimated at that time, because HL was not recognized as part of the AIDS spectrum. The increased incidence in HL is in the mixed cellularity subtype, which is typically Epstein-Barr virus (EBV) positive.
Distribution of lymphoma histotypes in individuals infected by HIV over 30 years in a European cohort (615 patients) compared with the CNICS USA cohort (476 patients)
| Histotype . | 1986-1995; London (158 patients) . | 1996-2005; London (200 patients) . | 2006-2015; London (257 patients) . | 1996-2000; CNICS (132 patients) . | 2001-2005; CNICS (201 patients) . | 2006-2010; CNICS (143 patients) . |
|---|---|---|---|---|---|---|
| BL | 3% | 10% | 20% ↑ | 7.6% | 10.9% | 16.8% ↑ |
| DLBCL | 63% | 59% | 37% ↓ | 43.9% | 45.8% | 35.7% ↓ |
| HL | 4% | 11% | 26% ↑ | 15.2% | 15.4% | 19.6% ↑ |
| PCNSL | 14.4% | 10.4% | 9.8% ↓ | |||
| PBL | 0 | 2% | 6% ↑ | |||
| PEL | 2% | 1% | 5% ↑ | |||
| Other | 18.9% | 17.4% | 18.2% |
| Histotype . | 1986-1995; London (158 patients) . | 1996-2005; London (200 patients) . | 2006-2015; London (257 patients) . | 1996-2000; CNICS (132 patients) . | 2001-2005; CNICS (201 patients) . | 2006-2010; CNICS (143 patients) . |
|---|---|---|---|---|---|---|
| BL | 3% | 10% | 20% ↑ | 7.6% | 10.9% | 16.8% ↑ |
| DLBCL | 63% | 59% | 37% ↓ | 43.9% | 45.8% | 35.7% ↓ |
| HL | 4% | 11% | 26% ↑ | 15.2% | 15.4% | 19.6% ↑ |
| PCNSL | 14.4% | 10.4% | 9.8% ↓ | |||
| PBL | 0 | 2% | 6% ↑ | |||
| PEL | 2% | 1% | 5% ↑ | |||
| Other | 18.9% | 17.4% | 18.2% |
Since the introduction of cART, the incidence of NHL has decreased by 50% mainly because of decreased PCNSL and the immunoblastic histologic subtype of DLBCL, consistent with CD4 counts. In contrast, the burden of HIV-associated BL and HL has increased16: pre-cART decade (1986-1995); early cART decade (1996-2005); late cART decade (2006-2015). European cohort26; CNICS USA cohort.25 ↑, increase of proportion in late cART decade; ↓, decrease of proportion in late cART decade.
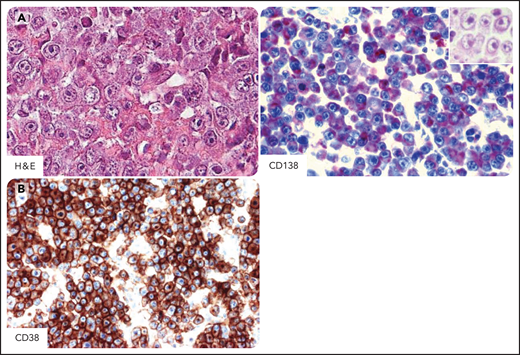
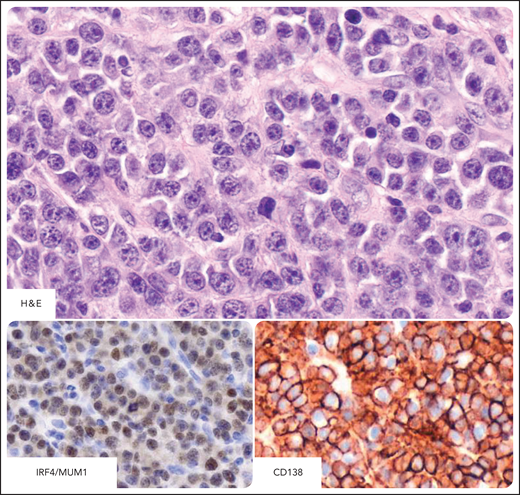
DLBCL in individuals infected by HIV. Systemic DLBCL with immunoblastic-plasmacytoid features, expressing the CD20+, CD10−, BCL6−, MUM1/IRF4+, CD138+, and CD38+ profile. (A) Hematoxylin-eosin stain (H&E) shows that most tumor cells display large cytoplasms and round or oval nuclei with large nucleoli. Tumor cells express cytoplasmic staining for CD138 (red). The H&E stain (inset) shows that the morphology of immunostained cells is immunoblastic. (B) Tumor cells express cytoplasmic and membranous staining for CD38 (brown). CD138 and CD38 immunohistochemistry, hematoxylin counterstain. Original magnification, ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
DLBCL in individuals infected by HIV. Systemic DLBCL with immunoblastic-plasmacytoid features, expressing the CD20+, CD10−, BCL6−, MUM1/IRF4+, CD138+, and CD38+ profile. (A) Hematoxylin-eosin stain (H&E) shows that most tumor cells display large cytoplasms and round or oval nuclei with large nucleoli. Tumor cells express cytoplasmic staining for CD138 (red). The H&E stain (inset) shows that the morphology of immunostained cells is immunoblastic. (B) Tumor cells express cytoplasmic and membranous staining for CD38 (brown). CD138 and CD38 immunohistochemistry, hematoxylin counterstain. Original magnification, ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
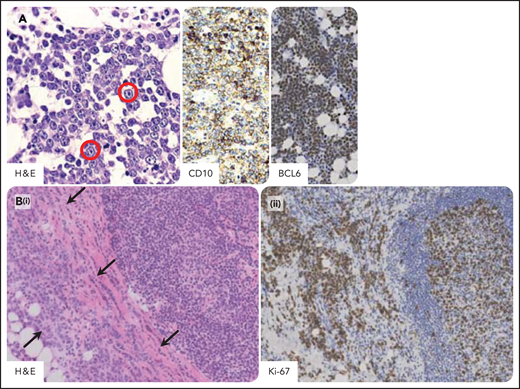
Burkitt lymphoma in individuals infected by HIV. Burkitt lymphoma with plasmacytoid differentiation; phenotypically, the tumor cells express B-cell antigens including CD20, CD10, and BCL6 and are consistently negative for BCL2. The proliferation index is very high, approximately 100%. (A) A homogeneous proliferation of medium-sized tumor cells displays a cohesive pattern. Tumor cells show round nuclei, multiple nucleoli, and small basophylic cytoplasm. Immunoblastic-like tumor cells (in the red circle) with larger nucleoli are also seen. Tumor cells typically express CD10 with membranous staining pattern and BCL6 with nuclear staining pattern. (B) Burkitt lymphoma involving capsular and extranodal tissues (arrows; i) and a hyperplastic lymphoid follicle within a reactive hyperplastic lymph node (ii). The extranodal pattern of tumor infiltration is confirmed by Ki-67 staining. H&E, hematoxylin-eosin stain; CD10, BCL6, and Ki-67 immunohistochemistry, hematoxylin counterstain. Original magnification, ×200. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 20×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
Burkitt lymphoma in individuals infected by HIV. Burkitt lymphoma with plasmacytoid differentiation; phenotypically, the tumor cells express B-cell antigens including CD20, CD10, and BCL6 and are consistently negative for BCL2. The proliferation index is very high, approximately 100%. (A) A homogeneous proliferation of medium-sized tumor cells displays a cohesive pattern. Tumor cells show round nuclei, multiple nucleoli, and small basophylic cytoplasm. Immunoblastic-like tumor cells (in the red circle) with larger nucleoli are also seen. Tumor cells typically express CD10 with membranous staining pattern and BCL6 with nuclear staining pattern. (B) Burkitt lymphoma involving capsular and extranodal tissues (arrows; i) and a hyperplastic lymphoid follicle within a reactive hyperplastic lymph node (ii). The extranodal pattern of tumor infiltration is confirmed by Ki-67 staining. H&E, hematoxylin-eosin stain; CD10, BCL6, and Ki-67 immunohistochemistry, hematoxylin counterstain. Original magnification, ×200. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 20×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
Main viral and molecular pathogenic pathways
The pathogenesis of hematologic malignancies in individuals infected by HIV is complex. HIV infection results in chronic B-cell activation both indirectly through altered cytokine production and directly through HIV proteins, including gp120, p17, and TAT.8,10,28,29 For example, patients receiving cART are still infected, with persistent production of viral proteins. The TAT protein appears to be possibly involved in the pathogenesis of BL.30
Different genetic alterations and viral infection occur in the pathogenetic pathways underlying the spectrum of lymphoproliferative disorders in individuals infected by HIV. Pathogenetic mechanisms in HIV-related lymphomagenesis specifically also include coinfection with the gammaherpesviruses EBV and Kaposi sarcoma-associated herpesvirus (KSHV).8,31 These 2 gammaherpesviruses escape the host's immunologic control, and cooperating with HIV seems to be crucial in promoting lymphomagenesis.
The pathogenetic pathway in BL (Figure 2) involves activation of MYC (100% of cases), inactivation of TP53 (50% to 60% of cases), and infection by EBV (30%-50% of cases).10,28 The pathogenesis of the 2 HIV-associated DLBCL variants (ie, centroblastic [CB] and immunoblastic [IB]; Figure 1) is more heterogeneous (see also below). Infection with EBV occurs in 90% of DLBCL with IB morphology and 30% of DLBCL with CB morphology. Many EBV-positive IB DLBCLs express the EBV-encoded transforming protein LMP-1. There is an association (20%) between molecular changes in the BCL6 proto-oncogene and HIV-associated CB DLBCL.10,28 Unclassifiable lymphomas with features intermediate between BL and DLBCL and high grade B-cell lymphoma with double or triple hit are overlapping categories and show rearrangements in BCL2 (and BCL6) and MYC genes.32 In addition to consistent infection by KSHV, PELs (Figure 3) are also commonly infected by EBV (80%).31 Molecular studies of cells in PEL have shown no rearrangements in BCL2, BCL6, and MYC genes.13 However, mutations in the BCL6 5' noncoding region are common in PEL.33 Regarding the molecular pathogenesis of PBL of the oral cavity type (Figure 4), EBV infection of the neoplastic clone has been frequently (70%) reported.16MYC translocation or amplification is observed in 50% of cases.16 The genomic landscape of plasmablastic lymphoma in HIV-positive individuals identify pervasive mutations in JAK-STAT3 and RAS-MAPK signaling pathways.34 Nearly 100% of cases of HL (Figure 5) are associated with EBV infection and express LMP-1.35 Specific genetic alterations are poorly known, although in EBV-infected HL, 9p24.1 amplification leading to programmed death-ligand 1 expression has been observed.36
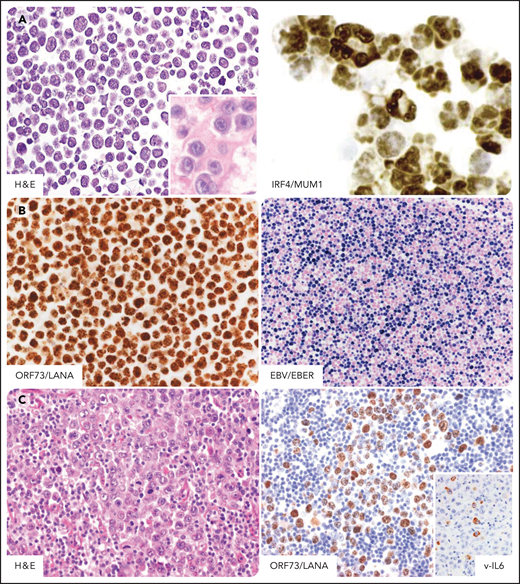
PEL in individuals infected by HIV. PEL cells express markers associated with plasma cell differentiation such as CD138, CD38, and MUM1/IRF4. Tumor cells are usually negative for B-cell and T-cell markers (data not shown). (A) In a cell line derived from a classic PEL, tumor cells display features bridging immunoblastic and anaplastic large cell lymphomas and morphologically show a certain degree of plasma cell differentiation. (Inset) In a cell block derived from a primary classic PEL tumor cell are features resembling immunoblastic lymphoma cells. Plasma cell differentiation is confirmed by nuclear immunohistochemical staining for IRF4/MUM1. (B) Immunohistochemical staining for ORF73/LANA detects evidence of KSHV infection. Typically, the staining pattern is speckled. EBER in situ hybridization detects Epstein-Barr virus in tumor cells. The positive nuclei are stained in blue. (C) In a case of extracavitary solid PEL, tumor cells show morphologic features similar to those seen in serous effusion of classic PEL. Immunohistochemical staining for ORF73/LANA is the standard assay to detect evidence of KSHV infection also in tumor tissue. (Inset) Fraction of tumor cells expresses KSHV viral IL6. H&E, hematoxylin-eosin stain; MUM1, ORF73/LANA, v-IL6, immunohistochemistry, hematoxylin counterstain; EBER, in situ hybridization, nuclear fast red counterstaining. Original magnification ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
PEL in individuals infected by HIV. PEL cells express markers associated with plasma cell differentiation such as CD138, CD38, and MUM1/IRF4. Tumor cells are usually negative for B-cell and T-cell markers (data not shown). (A) In a cell line derived from a classic PEL, tumor cells display features bridging immunoblastic and anaplastic large cell lymphomas and morphologically show a certain degree of plasma cell differentiation. (Inset) In a cell block derived from a primary classic PEL tumor cell are features resembling immunoblastic lymphoma cells. Plasma cell differentiation is confirmed by nuclear immunohistochemical staining for IRF4/MUM1. (B) Immunohistochemical staining for ORF73/LANA detects evidence of KSHV infection. Typically, the staining pattern is speckled. EBER in situ hybridization detects Epstein-Barr virus in tumor cells. The positive nuclei are stained in blue. (C) In a case of extracavitary solid PEL, tumor cells show morphologic features similar to those seen in serous effusion of classic PEL. Immunohistochemical staining for ORF73/LANA is the standard assay to detect evidence of KSHV infection also in tumor tissue. (Inset) Fraction of tumor cells expresses KSHV viral IL6. H&E, hematoxylin-eosin stain; MUM1, ORF73/LANA, v-IL6, immunohistochemistry, hematoxylin counterstain; EBER, in situ hybridization, nuclear fast red counterstaining. Original magnification ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
PBL of the oral cavity type in individuals infected by HIV. PBL tumor cells express markers associated with plasma cell differentiation such as CD138, CD38, and MUM1/IRF4. Tumor cells are usually negative for CD20, CD45, and CD56 (data not shown). H&E stain shows that the lymphoproliferation consists of medium-sized tumor cells displaying a cohesive pattern. Tumor cells show round nuclei with multiple nucleoli or few prominent nucleoli. The cytoplasm is generally large and basophilic. Immunostaining with the plasma cell markers MUM1/IRF4 and CD138 is strong in PBL. The pattern of staining is nuclear for IRF4/MUM1 and cytoplasmic and membranous for CD138. MUM1 and CD138, immunohistochemistry, hematoxylin counterstain. Original magnification, ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop 6.
PBL of the oral cavity type in individuals infected by HIV. PBL tumor cells express markers associated with plasma cell differentiation such as CD138, CD38, and MUM1/IRF4. Tumor cells are usually negative for CD20, CD45, and CD56 (data not shown). H&E stain shows that the lymphoproliferation consists of medium-sized tumor cells displaying a cohesive pattern. Tumor cells show round nuclei with multiple nucleoli or few prominent nucleoli. The cytoplasm is generally large and basophilic. Immunostaining with the plasma cell markers MUM1/IRF4 and CD138 is strong in PBL. The pattern of staining is nuclear for IRF4/MUM1 and cytoplasmic and membranous for CD138. MUM1 and CD138, immunohistochemistry, hematoxylin counterstain. Original magnification, ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop 6.
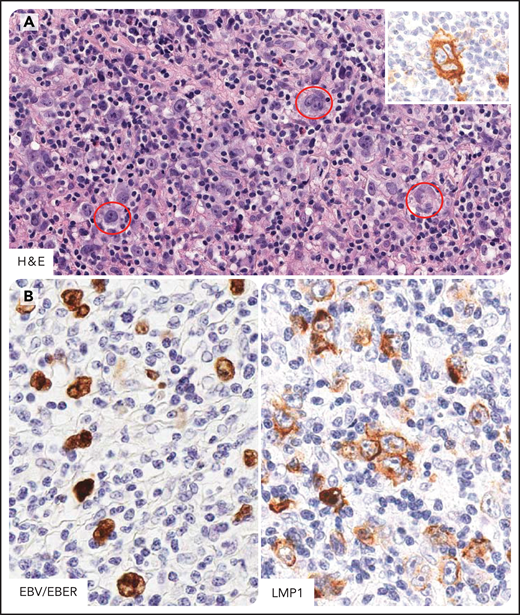
HL in individuals infected by HIV. Hodgkin and Reed-Sternberg cells (HRS) in HL of individual infected by HIV express CD15, CD30, CD40, and MUM1/IRF4 and LMP1 (EBV-type II latency) (A) Several HRS cells are seen (in the red circle) within a mixed inflammatory microenvironment. These cells express CD30 (inset). (B) EBV-infected tumor cells are demonstrated by EBER in situ hybridization and LMP1 immunostaining. CD30 and LMP1, immunohistochemistry, hematoxylin counterstain; EBER, in situ hybridization, nuclear fast red counterstaining. Original magnification, ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop 6.
HL in individuals infected by HIV. Hodgkin and Reed-Sternberg cells (HRS) in HL of individual infected by HIV express CD15, CD30, CD40, and MUM1/IRF4 and LMP1 (EBV-type II latency) (A) Several HRS cells are seen (in the red circle) within a mixed inflammatory microenvironment. These cells express CD30 (inset). (B) EBV-infected tumor cells are demonstrated by EBER in situ hybridization and LMP1 immunostaining. CD30 and LMP1, immunohistochemistry, hematoxylin counterstain; EBER, in situ hybridization, nuclear fast red counterstaining. Original magnification, ×400. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop 6.
In the case of HIV, cooperation with KSHV multiple lesions including MCD, PEL, and KS can occur in a single patient, either simultaneously or at different times.37 KSHV infection is consistently associated with PEL, KSHV-associated MCD, and a KSHV inflammatory cytokine syndrome (KICS).13,38,39 A high percentage of patients with KICS have KS or PEL. KSHV-derived viral interleukin-6 (vIL6) may contribute to the pathogenesis of PEL, KS, and MCD.8 In 80% of cases of PEL, tumor cells are coinfected by EBV, making PEL a unique model of oncogenic viral cooperation occurring within the same target cell.31 KICS, a syndrome characterized by severe MCD-like inflammatory symptoms without Castleman disease exhibit elevation of vIL6 and may represent a prodrome to KSHV-associated MCD or PEL.39-41
Table 2 shows the phenotypic and genotypic features and viral association of lymphomas in individuals infected by HIV.
Lymphomas in individuals infected by HIV: pathologic spectrum, immunophenotypic markers, virologic association, and genetic features10,18
| . | CD20 . | BCL6 . | IRF4/MUM1 . | CD138 . | Other positive cell markers . | EBV infection (frequency) . | EBV latency . | KSHV infection . | Genetic features . |
|---|---|---|---|---|---|---|---|---|---|
| BL–plasmacytoid | Positive | Positive | Negative | Negative | CD10, MYC | Positive (60%) | I | Negative | Myc rearrangement and point mutation; TP53 point mutation |
| DLBCL, immunoblastic– plasmacytoid | Positive (may be lost) | Negative | Positive | Positive | CD10, CD30, CD5 | Positive (100%) | II/III | Negative | TP53 point mutation; Myc rearrangement (minority); RAS point mutation; BCL2 and BCL6 rearrangements |
| DLBCL, centroblastic | Positive | Positive | Negative | Negative | CD10, CD5 | Positive (25%) | — | Negative | |
| PBL of the oral cavity type | Positive (may be lost) | Negative | Positive | Positive | CD10, CD31, CD38, CD56 | Positive (70%) | 0/I | Negative | Myc rearrangement; P53 overexpression |
| Classic PEL | Negative | Negative | Positive | Positive | CD30, CD31, CD71, EMA | Positive (80-100%) | I | Positive (100%) | Complex karyotype, no recurrent translocation, Tp53 and RAS rarely mutated |
| Solid PEL | Negative | Negative | Positive | Positive | CD30, EMA | Positive (80-100%) | I | Positive (100%) | Occasional p53 positive cells |
| MCD-associated large cell lymphoma* | Positive (may be lost) | Negative | Positive | Positive | Λ light chiain, CD45 | Negative | — | Positive (100%) | Myc rearrangement, TP53 point mutation |
| HL | Negative | Negative | Positive | NR | CD15, CD30, CD40 | Positive (80-100%) | II | Negative | NR |
| . | CD20 . | BCL6 . | IRF4/MUM1 . | CD138 . | Other positive cell markers . | EBV infection (frequency) . | EBV latency . | KSHV infection . | Genetic features . |
|---|---|---|---|---|---|---|---|---|---|
| BL–plasmacytoid | Positive | Positive | Negative | Negative | CD10, MYC | Positive (60%) | I | Negative | Myc rearrangement and point mutation; TP53 point mutation |
| DLBCL, immunoblastic– plasmacytoid | Positive (may be lost) | Negative | Positive | Positive | CD10, CD30, CD5 | Positive (100%) | II/III | Negative | TP53 point mutation; Myc rearrangement (minority); RAS point mutation; BCL2 and BCL6 rearrangements |
| DLBCL, centroblastic | Positive | Positive | Negative | Negative | CD10, CD5 | Positive (25%) | — | Negative | |
| PBL of the oral cavity type | Positive (may be lost) | Negative | Positive | Positive | CD10, CD31, CD38, CD56 | Positive (70%) | 0/I | Negative | Myc rearrangement; P53 overexpression |
| Classic PEL | Negative | Negative | Positive | Positive | CD30, CD31, CD71, EMA | Positive (80-100%) | I | Positive (100%) | Complex karyotype, no recurrent translocation, Tp53 and RAS rarely mutated |
| Solid PEL | Negative | Negative | Positive | Positive | CD30, EMA | Positive (80-100%) | I | Positive (100%) | Occasional p53 positive cells |
| MCD-associated large cell lymphoma* | Positive (may be lost) | Negative | Positive | Positive | Λ light chiain, CD45 | Negative | — | Positive (100%) | Myc rearrangement, TP53 point mutation |
| HL | Negative | Negative | Positive | NR | CD15, CD30, CD40 | Positive (80-100%) | II | Negative | NR |
Other histotypes (rare) include the same lymphomas that develop in the absence of HIV infection: primary central nervous system lymphoma; lymphomas of the marginal zone; high grade B-cell lymphoma; polymorphic B-cell lymphoma (PTLD-like); plasmacytoma; peripheral T-cell lymphoma, NOS. NR, not reported. Modified and adapted from Carbone et al.18
MCD-associated large cell lymphoma should be defined on the basis of molecular clonality and not only based on the Λ phenotype.
Stage of differentiation
Figure 6 shows the correlation of the histologic spectrum of lymphomas of individuals infected by HIV with the putative cell of origin and indicates that DLBCL with IB morphology, PEL and the extracavitary variant (Figure 3), and PBL of the oral cavity type (Figure 4) show immunoblast-like tumor cells with plasmacytic differentiation.31
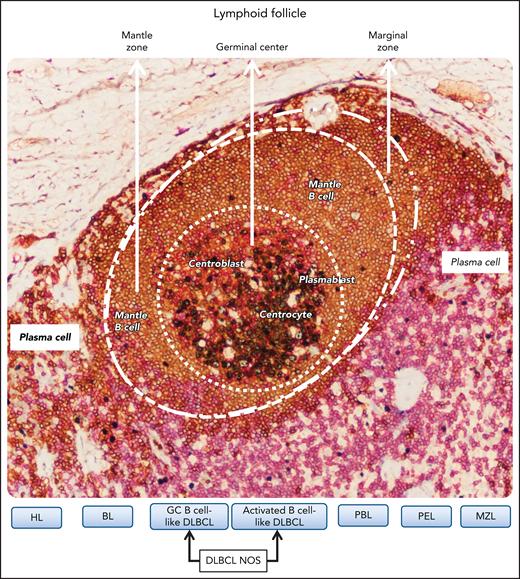
Relationship between B-cell lymphomas in individual infected by HIV and B-cell differentiation stage related to the different zones of the lymphoid follicle. B-cell lymphomas putatively develop from the malignant transformation of B cells at various stages of differentiation. The definition of cell of origin (COO) starts from the assumption that B-cell lymphomas are frozen at a given B-cell differentiation stage associated with different zones of the lymphoid follicle. The figure shows a lymphoid follicle of a reactive lymph node stained by multiplex immunostaing. In the different zones of the follicle B lymphocytes express CD20 (brown). In the germinal center (GC), B lymphocytes also express Ki67 (gray), whereas outside the marginal zone of the follicle, T lymphocytes express CD3. CD3-positive cells are also scattered in the mantle zone and in the GC. Regarding lymphomas, HL corresponds to GC B cells, and BL tumor cells correspond to centroblasts. GC B cell–like DLBCL corresponds to B cells that are arrested at various stages of the GC transits and the activated B cell-like DLBCL seems to derive from GC B cells evolving to plasma cell differentiation. PBL and PEL seem to derive from GC B cells resembling plasmablasts. Marginal zone lymphoma (MZL) corresponds to marginal zone B cells. Original magnification, ×200. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 20×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
Relationship between B-cell lymphomas in individual infected by HIV and B-cell differentiation stage related to the different zones of the lymphoid follicle. B-cell lymphomas putatively develop from the malignant transformation of B cells at various stages of differentiation. The definition of cell of origin (COO) starts from the assumption that B-cell lymphomas are frozen at a given B-cell differentiation stage associated with different zones of the lymphoid follicle. The figure shows a lymphoid follicle of a reactive lymph node stained by multiplex immunostaing. In the different zones of the follicle B lymphocytes express CD20 (brown). In the germinal center (GC), B lymphocytes also express Ki67 (gray), whereas outside the marginal zone of the follicle, T lymphocytes express CD3. CD3-positive cells are also scattered in the mantle zone and in the GC. Regarding lymphomas, HL corresponds to GC B cells, and BL tumor cells correspond to centroblasts. GC B cell–like DLBCL corresponds to B cells that are arrested at various stages of the GC transits and the activated B cell-like DLBCL seems to derive from GC B cells evolving to plasma cell differentiation. PBL and PEL seem to derive from GC B cells resembling plasmablasts. Marginal zone lymphoma (MZL) corresponds to marginal zone B cells. Original magnification, ×200. Images were taken using a Nikon Eclipse 80i microscope with a Plan Fluor 20×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
Table 3 shows the correlation of the cell of origin with clinical outcome in these lymphomas as reported in the current literature.17,28 Gene expression profiling of DLBCLs in patients of the general population has identified molecular subgroups, which correlate with prognosis.42 At least 2 major subgroups of DLBCL establishing a putative cell of origin could be identified. One resembles CB germinal center B cells (GC B-cell like) and the other resembles IB/activated B cells (activated B-cell like).42 A significantly worse prognosis for the activated B cells subtype has been demonstrated.42-44 However, no consensus has emerged in the HIV setting for distribution and relation to outcome of biologically distinct subtypes of DLBCL, germinal center B-cells, and activated B-cells.45-48
Histogenetic subclassification of lymphomas in HIV-infected individuals and clinical outcome reported in the current literature in cART era
| Histotype . | GC . | Post-GC . | ||||
|---|---|---|---|---|---|---|
| GC B-cell type . | Activated B-cell type . | Plasmablastic type/plasma cell type . | ||||
| BL . | DLBCL-CB . | DLBCL-IB . | PBL . | PEL . | KSHV + MCD . | |
| Phenotypic features | ||||||
| CD10 | + | + | −/+ | −/+ | − | − |
| BCL-6 | + | + | − | − | − | − |
| MUM1 | − | − | + | + | + | + |
| CD138 | − | − | −/+ | +/− | + | +/− |
| Immunodeficiency | From mild————————————————————————————————–→ to severe | |||||
| Outcome | Improved outcome in cART era | Improved outcome in cART era | Improved outcome in cART era | Poor prognosis | Poor prognosis | Poor prognosis |
| Histotype . | GC . | Post-GC . | ||||
|---|---|---|---|---|---|---|
| GC B-cell type . | Activated B-cell type . | Plasmablastic type/plasma cell type . | ||||
| BL . | DLBCL-CB . | DLBCL-IB . | PBL . | PEL . | KSHV + MCD . | |
| Phenotypic features | ||||||
| CD10 | + | + | −/+ | −/+ | − | − |
| BCL-6 | + | + | − | − | − | − |
| MUM1 | − | − | + | + | + | + |
| CD138 | − | − | −/+ | +/− | + | +/− |
| Immunodeficiency | From mild————————————————————————————————–→ to severe | |||||
| Outcome | Improved outcome in cART era | Improved outcome in cART era | Improved outcome in cART era | Poor prognosis | Poor prognosis | Poor prognosis |
Histogenetic lymphomas subclassification27,28; reported clinical outcomes.27,28 BL, Burkitt lymphoma; DLBCL-CB, diffuse large B-cell lymphoma, centroblastic variant; DLBCL-IB, diffuse large B-cell lymphoma, immunoblastic variant; PBL, plasmablastic lymphoma; PEL, primary effusion lymphoma; –, absent; +, present; +/− usually present; −/+, usually absent.
Lymphomas in individuals infected by HIV are, in terms of cellular derivation, less heterogeneous than lymphomas that develop in the absence of HIV infection.31 Most DLBCL arising in individuals infected by HIV are characterized by an immunoblastic plasmacytoid morphology and expression of CD138 and MUM1/IRF4 (Figure 1), markers of post-CG/terminal B-cell/plasmacytic differentiation (Figure 6). An immunohistochemical study showed that the expression of BCL6 (a GC marker) and MUM1/IRF4 (a post-GC marker) were mutually exclusive in HIV-associated lymphomas. BCL6 was generally restricted to the CB and MUM1/IRF4 to the IB variants of DLBCL.27,28
Change in treatment paradigm
Table 4 shows the major clinical features of the common types of lymphoma and MCD in individuals infected by HIV. HIV-associated NHL is characterized by advanced-stage disease, B symptoms, extranodal involvement at diagnosis, and aggressive clinical course.17,18
Major clinical features and laboratory findings in lymphomas and MCD occurring in individuals infected by HIV
| Histotype . | Common presentation . | CD4/μL at diagnosis . | Clinical features . |
|---|---|---|---|
| BL | Nodal, extranodal, bone marrow | >200* | Increasing prevalence in cART era Improved outcomes in cART era Only in EBV+, immunoblastic plasmacytoid morphology |
| DLBCL† | Nodal and extranodal | <200 | The most common lymphoma Late manifestation of HIV infection May have CNS involvement Improved outcome in cART era |
| PEL | Effusions; nodal and extranodal presentations are found (solid PEL) | <100 | Concurrent Kaposi sarcoma common Aggressive behavior Poor prognosis |
| PBL | Extranodal, oral cavity or other extranodal or nodal sites | <200 | Aggressive behavior Poor prognosis |
| MCD-associated large cell lymphoma | Extranodal and nodal | >200 | Aggressive behavior Poor prognosis |
| MCD | Nodal | >200 | Severe B symptoms Increased KSHV viral load and elevated levels of circulating vIL6, h-IL6, and IL10 |
| HL | Nodal and extranodal | ≈200 | Increased incidence over time (SIR 22) Good prognosis |
| Histotype . | Common presentation . | CD4/μL at diagnosis . | Clinical features . |
|---|---|---|---|
| BL | Nodal, extranodal, bone marrow | >200* | Increasing prevalence in cART era Improved outcomes in cART era Only in EBV+, immunoblastic plasmacytoid morphology |
| DLBCL† | Nodal and extranodal | <200 | The most common lymphoma Late manifestation of HIV infection May have CNS involvement Improved outcome in cART era |
| PEL | Effusions; nodal and extranodal presentations are found (solid PEL) | <100 | Concurrent Kaposi sarcoma common Aggressive behavior Poor prognosis |
| PBL | Extranodal, oral cavity or other extranodal or nodal sites | <200 | Aggressive behavior Poor prognosis |
| MCD-associated large cell lymphoma | Extranodal and nodal | >200 | Aggressive behavior Poor prognosis |
| MCD | Nodal | >200 | Severe B symptoms Increased KSHV viral load and elevated levels of circulating vIL6, h-IL6, and IL10 |
| HL | Nodal and extranodal | ≈200 | Increased incidence over time (SIR 22) Good prognosis |
CD4 counts are often normal in patients with BL, and BL often presents when there is no overt evidence of immune suppression.
Primary central nervous system lymphoma, which is nearly EBV+ in the setting of HIV, should be distinguished from other DLBCL.
Treatment of HIV lymphomas has evolved over time, in line with better control of HIV replication and recovery of immune function by cART.25,49 The overall survival (OS) for these patients has dramatically improved in the modern cART era, with a 5-year survival increased from 13% in the pre-cART era (1986-1995) to 70% in the late cART era (2006-2015).26 Outcomes are now determined by NHL disease features (ie, age-adjusted international prognostic [IPI] score and lack of complete response [CR] to therapy rather than HIV-specific factors: CD4 count, prior AIDS-defining illness, HIV viral load).50 However, disparities in practice pattern based on HIV status may influence outcomes in this population that is still excluded from prospective clinical trials.51 Data from large population-based studies have shown that HIV infection remains associated with higher risk of death in patients with all lymphoma subtypes compared with general population.52,53 Major provocative questions on HIV-associated NHL treatment are reported in Table 5. Dissemination of effective management strategies is mandatory to improve patient outcome.
Open questions on new treatment strategies of lymphomas in individuals infected by HIV
| Questions . | Comments . |
|---|---|
| 1. Should rituximab be included within the regimen in CD20+ HIV-NHL? | The combination of rituximab and chemotherapy results in significant clinical benefit for all CD20+ HIV-NHL patients compared with chemotherapy alone (higher CR rates and better PFS and OS), as in the general population. There is need to maximize opportunistic infection prophylaxis in patients with CD4 count ≤50/µL, according to current guidelines on HIV management. |
| 2. What is the best chemotherapy regimen (infusional vs bolus chemotherapy)? | In the cART era, the standard treatments of HIV-associated lymphomas (infusional or bolus chemotherapy) mirror that of HIV-negative patients, but controversy remains regarding the optimal chemotherapy regimen. |
| 3. Are there subtypes of lymphomatous disease that should be treated differently from others? | The best therapy for HIV-associated BL still remains unclear, but CHOP chemotherapy backbone is not recommended. HIV-infected patients with PBL or PEL continue to be characterized by a dismal prognosis and should be included in clinical trials. HD methothrexate-based chemotherapy is recommended for selected† HIV-positive patients with PCNSL, based on general consensus. For patients inelegible for chemotherapy, WBRT remains a useful palliative treatment. |
| 4. Should antiretroviral therapy be suspended during chemotherapy? | All HIV-infected patients with cancer must be mainteined on cART during antineoplastic treatment. Ritonavir or cobicistat-based antiretroviral regimens must be avoided because of drug-drug interactions. |
| 5. Is the approach with intensive chemotherapy and peripheral stem cell rescue feasible? | HIV infection should not preclude lymphoma patients from undergoing HDC-ASCT, according to the same eligibility criteria adopted for the general population. |
| Questions . | Comments . |
|---|---|
| 1. Should rituximab be included within the regimen in CD20+ HIV-NHL? | The combination of rituximab and chemotherapy results in significant clinical benefit for all CD20+ HIV-NHL patients compared with chemotherapy alone (higher CR rates and better PFS and OS), as in the general population. There is need to maximize opportunistic infection prophylaxis in patients with CD4 count ≤50/µL, according to current guidelines on HIV management. |
| 2. What is the best chemotherapy regimen (infusional vs bolus chemotherapy)? | In the cART era, the standard treatments of HIV-associated lymphomas (infusional or bolus chemotherapy) mirror that of HIV-negative patients, but controversy remains regarding the optimal chemotherapy regimen. |
| 3. Are there subtypes of lymphomatous disease that should be treated differently from others? | The best therapy for HIV-associated BL still remains unclear, but CHOP chemotherapy backbone is not recommended. HIV-infected patients with PBL or PEL continue to be characterized by a dismal prognosis and should be included in clinical trials. HD methothrexate-based chemotherapy is recommended for selected† HIV-positive patients with PCNSL, based on general consensus. For patients inelegible for chemotherapy, WBRT remains a useful palliative treatment. |
| 4. Should antiretroviral therapy be suspended during chemotherapy? | All HIV-infected patients with cancer must be mainteined on cART during antineoplastic treatment. Ritonavir or cobicistat-based antiretroviral regimens must be avoided because of drug-drug interactions. |
| 5. Is the approach with intensive chemotherapy and peripheral stem cell rescue feasible? | HIV infection should not preclude lymphoma patients from undergoing HDC-ASCT, according to the same eligibility criteria adopted for the general population. |
HDC-ASCT, high-dose chemotherapy-autologous stem cell transplantation.
Patients responsive to cART, with good performance status and without opportunistic infections.
Should rituximab be included within the regimen in CD20-positive HIV NHL?
The anti-CD20 monoclonal antibody rituximab (R) results in significant benefit when used with chemotherapy for treatment of aggressive B-cell NHL of the general population, whereas its role in HIV-associated DLBCL has been controversial.54,55 An AIDS Malignancy Consortium (AMC) randomized phase 3 study with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) with or without rituximab found that rituximab was associated with only a trend in improved outcome, but with a significant increase in infectious deaths (14% vs 2%, P < .001), particularly in patients with CD4 count <50/µL and in those who received rituximab maintanance,56 which has not been shown to be useful in HIV-negative NHL. Subsequent phase 2 studies demonstrated that the combination of R-CHOP, R-CDE (cyclophosphamide, doxorubicin, etoposide),54,57,58 R-EPOCH or dose-adjusted (DA)-EPOCH-R (etoposide, prednisone, vincristine, doxorubicin and cyclophosphamide dose adjusted to CD4 count) plus rituximab were beneficial (CR rate of 69%-91%, 2-year survival rate of 62%-75%) with low infectious death rate (≤10%)47,59,60 (Table 6). In a pooled analysis of 1546 patients, rituximab was associated with higher CR rate (odd ratio, 2.89; P < .001), improved progression free survival (PFS; hazard ratio [HR], 0.50; P < .001), and OS (HR, 0.51; P < .0001).61
Pivotal clinical trials with rituximab (R) and chemotherapy in HIV-associated NHL
| . | R-CHOP . | R-CDE . | R-EPOCH . | SC-EPOCH-RR . | ||
|---|---|---|---|---|---|---|
| Kaplan et al56 . | Boué et al57 . | Ribera et al58 . | Spina et al54 . | Sparano et al59 . | Dunleavy et al47 . | |
| No. of patients | 150 (99 R arm) | 61 | 95 | 74 | 106 (51 cR arm) | 33 |
| Study design | Phase 3: R-CHOP-R vs CHOP | Phase 2 | Phase 2 | Pooled 3 phase 2 trials | Phase 2 randomized: R-EPOCH vs EPOCH-R° | Phase 2 |
| CD4 µL median | 130 | 172 | 158 | 161 | 194 | 208 |
| Histology, % | ||||||
| DLCL BL | 81 8 | 72 26 | 81 8 | 72 28 | 80 20 | 100 0 |
| aa-IPI ≥2% | 43 | 48 | 58 | 57 | 64 | 76 |
| Outcome, % | ||||||
| CR rate | 58 vs 47 | 77 | 69 | 70 | 73 vs 55* | 91 |
| PFS | 11.3 vs 9.5 mo | 69 (2 y) | NA | 52 EFS (2 y) | 66 vs 63 (2 y) | 84 (5 y) |
| OS | 28 vs 35 mo | 75 (2 y) | 56 (3 y) | 64 (2 y) | 70 vs 67 (2 y) | 68 (5 y) |
| Infectious deaths, % | 14° vs 2* | 2 | 7 | 7 | 10° vs 7 | 0 |
| . | R-CHOP . | R-CDE . | R-EPOCH . | SC-EPOCH-RR . | ||
|---|---|---|---|---|---|---|
| Kaplan et al56 . | Boué et al57 . | Ribera et al58 . | Spina et al54 . | Sparano et al59 . | Dunleavy et al47 . | |
| No. of patients | 150 (99 R arm) | 61 | 95 | 74 | 106 (51 cR arm) | 33 |
| Study design | Phase 3: R-CHOP-R vs CHOP | Phase 2 | Phase 2 | Pooled 3 phase 2 trials | Phase 2 randomized: R-EPOCH vs EPOCH-R° | Phase 2 |
| CD4 µL median | 130 | 172 | 158 | 161 | 194 | 208 |
| Histology, % | ||||||
| DLCL BL | 81 8 | 72 26 | 81 8 | 72 28 | 80 20 | 100 0 |
| aa-IPI ≥2% | 43 | 48 | 58 | 57 | 64 | 76 |
| Outcome, % | ||||||
| CR rate | 58 vs 47 | 77 | 69 | 70 | 73 vs 55* | 91 |
| PFS | 11.3 vs 9.5 mo | 69 (2 y) | NA | 52 EFS (2 y) | 66 vs 63 (2 y) | 84 (5 y) |
| OS | 28 vs 35 mo | 75 (2 y) | 56 (3 y) | 64 (2 y) | 70 vs 67 (2 y) | 68 (5 y) |
| Infectious deaths, % | 14° vs 2* | 2 | 7 | 7 | 10° vs 7 | 0 |
P < .005; majority of patients with CD4 count <50/µL and without combination antiretroviral therapy.
One area of uncertainty remains on the use of rituximab in patients with CD4 count ≤50/µL.55 Treatment outcomes significantly improved for patients with NHL with CD4 count <50/µL enrolled in clinical trials in the contemporary (2005-2010) cART era when the 2-year OS reached 65% from 16% in the pre-cART era.60 Moreover, an observational study showed that rituximab was not associated with a higher risk of infectious deaths in patients with low CD4 count (<100/µL).62 There is now general consensus to use rituximab in all patients with HIV-associated CD20-positive NHL,18 maximizing supportive care, particularly opportunistic infection prophylaxis, in high-risk patients (https://aidsinfo.nih.gov/guidelines/html/4/adult and adolescent-oi-prevention-and treatment guidelines).
A second area of uncertainty remains on the use of rituximab in patients with active KS and very low CD4 cell count even if the use of anthracycline-based chemotherapy may avoid rituximab-induced KS progression, as reported in patients with KS and MCD.63
What is the best chemotherapy regimen for HIV-NHL; is infusional chemotherapy superior to bolus regimens?
Rationale for infusional chemotherapy, such as CDE and EPOCH or its variants47,64 included evidence of less tumor resistance, less cardiac toxicity with prolonged doxorubicin administration, and better therapeutic synergy with rituximab.65 An AMC pooled analysis has suggested that infusional R-EPOCH may be more effective than bolus R-CHOP in patients with HIV-associated aggressive B-cell NHL. Improved event-free survival (EFS; HR, 0.40; P < .001) and OS (HR, 0.38; P < .01) were observed in both high-risk and low-risk patients but were more pronounced in the high-risk IPI group.60 In contrast, a randomized trial in immunocompetent patients with DLBCL showed that DA-EPOCH-R and R-CHOP were equally effective but with greater toxicity and complexity of infusional therapy. Whether patients with high-risk IPI may benefit from DA-EPOCH-R remains unknown because of the small subset size and statistical caveats of this study.66
Are there subtypes of lymphomatous disease that should be treated differently from others?
BL
There is evidence that the prognosis for HIV-infected patients with BL has improved only in the contemporary cART era, with 2-year OS ranging from 45% in the early era (1996-2000) to 75% in the late (≥2005) cART era.50 Use of intensive chemotherapy regimens may have contributed to the more favorable outcome. However, major concern of these regimens remains treatment-related toxicity.
Dose-intensive regimens as CODOX-M/IVAC (cyclophosphamide, doxorubicin, vincristine, methotrexate, etoposide, ifofosfamide, cytarabine) or CVAD/HD-MTX (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone followed by high-dose methotrexate) resulted in CR rates between 63% and 92%. However, toxicity was high with 11% to 15% treatment-related deaths. In contrast, modification of CODOX-M/IVAC regimen in combination with rituximab resulted in a 2-year survival rate of 69%, with only 1 treatment-related death and lower grade 3 to 4 toxicity (79% vs 100%) compared with the parent regimen.67,68 An alternative regimen for patients with BL without central nervous system (CNS) involvement may be risk-adapted DA-EPOCH-R, resulting in a 4-year EFS of 85%, regardless of HIV status.69 To date, the best therapy for HIV-associated BL still remains unclear, but CHOP chemotherapy backbone is not recommended.
PBL and PEL
The optimal treatment of patients with PBL or PEL is not yet defined, as clinical trials are lacking. In retrospective series, PBL showed disappointing outcomes with CHOP or CHOP-like regimens and with dose-intensive chemotherapy. Despite a 69% CR rate to chemotherapy, the 3-year OS remains at 25%.70 Bortezomib in combination with EPOCH has demonstrated activity in a retrospective series, with a CR rate of 91% and 5-year OS of 65%,71 but these data need to be confirmed by prospective studies.
In the cART era, PEL continues to be characterized by a dismal prognosis, with a 5-year OS of 28%, highlighting the inadequacy of currently standard treatment.72 Promising results have been recently reported with EPOCH-based regimen (3-year EFS, 71%), but data need to be confirmed in a larger series.73
PCNSL
In the cART era, PCNSL is a rare NHL with poor prognosis and typically remains associated with severe CD4 T-cell lymphopenia. Unlike HIV-negative PCNSL, HIV-positive PCNSL is EBV positive and tends to present with multiple brain lesions. Whole brain radiation therapy (WBRT) was used extensively as the only therapy in the pre-cART era, with poor outcomes being OS of only 3 to 4 months.55 Retrospective studies showed the feasibility and efficacy of high-dose (HD) methothrexate plus cART with long-term OS.74-76 A phase 2 study has recently shown a 67% CR rate for cART and rituximab HD methotrexate in 12 patients with PCNSL with a 5-year survival of 67% and preservation or improvement of neurocognitive function.77 Based on general consensus, current guidelines recommended HD methothrexate–based chemotherapy for patients responsive to cART, with good performance status and without opportunistic infections.75 For patients ineligible for chemotherapy, WBRT remains a useful palliative treatment.
Should antiretroviral therapy be suspended during chemotherapy?
The question of whether to use cART concomitantly or just after chemotherapy is because of potential risk of adverse drug–drug interactions, especially when strong cytochrome 3A4 inhibitors such as ritonavir or cobicistat-based antiretroviral regimens are used.78 Concurrent cART has been associated with improved CR rate61 and improved immunorecovery after chemotherapy.79 Moreover, many new antiretrovirals with fewer drug interactions are now available, such as integrase strand-transfer inhibitor without cobicistat (raltegravir, dolutegravir, and bictegravir). All HIV-infected patients with cancer must be maintained on cART during antineoplastic treatment, preferably with these integrase strand-transfer inhibitor–based regimens.
Is the approach with intensive chemotherapy and peripheral stem cell rescue feasible?
HIV infection has long been considered a controindication to HD chemotherapy with autologous stem cell transplantation. Since 2000, several prospective studies have demonstrated the safety and efficacy of this treatment strategy in HIV-positive relapsed/refractory lymphomas, with 3-year OS ranging from 48% to 90% and low treatment-related mortality (5%-8%).80,81 In retrospective case-control studies, outcomes between HIV-infected patients and controls were not statistically different.82 However, data on long-term HIV-positive survivors affected by relapsed/refractory lymphoma undergoing HD/ASCT support the need of active surveillance of opportunistic infections (35%) early after HD/ASCT and second cancers (19%) later from ASCT.83
Allogenic hematopoietic cell transplant (alloHCT) is the emerging treatment modality for selected HIV-infected patients with different hematologic disorders including refractory lymphomas.84,85 In 1 small phase 2 study, the 1-year nonrelapsed mortality rate was 12%, the 1-year OS was 59%, and complete donor chimerism was 69% at 6 months. However, alloHCT was limited by the risk of graft-versus-host disease (grade 2-4, 44%), severe infectious complications (47%), and unexpected adverse events (82%).84 In contrast, there are 2 cases of virologic cure of HIV after alloHCT using CCR5Δ32 homozygous donors.86,87
HL
HL (Figure 5) incidence is growing among HIV-infected patients during the first months on cART, suggesting that the disease may be unmasked by the immune reconstitution inflammatory syndrome.88-90 HIV-associated HL is, in most cases, very different from that observed in the general population91: it is systemic, sometimes even without lymph nodes involvement and EBV positive. A high proportion of cases are of mixed cellularity subtype. The inference is that one probably requires a more intact immune system to develop HL in contrast to EBV-positive DLBCL of the immunoblastic subtype.92 Moreover, with less severe immunosuppression, the nodular sclerosis subtype becomes more frequent.18
Patients typically present moderate immune deficiency, B symptoms, and advanced stages involving bone marrow, liver, and spleen.18 Primary bone marrow HL was found in 3% to 14% of cases and was characterized by an aggressive clinical course.18
The combination of cART and chemotherapy has resulted in remarkable outcome improvements in patients with HIV-associated HL enrolled in observational cohorts.93-95 In contrast, population-based studies demonstrated poor prognosis because of disparity in treatment delivery.25,96
ABVD (adriamycin; ie, doxorubicin, bleomycin, vinblastine, dacarbazine) regimen plus cART with or without radiation therapy is the standard of care in patients with HIV-associated HL,18,97 resulting in CR rates of 74% to 87% and 5-year OS of 76% to 81%.93-95 In a German study, stage- and risk-adapted treatment including ABVD with involved field radiation therapy in early stages and ABVD or BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisolone) in advanced stages were feasible and effective. CR rates were 96% for early favorable stages, 100% for early unfavorable stages, and 86% for advanced stages. The 2-year OS rate was 91%, with no significant differences between the stage groups.98 However, BEACOPP was toxic, dose reductions/delays occurred in >50% of patients, and treatment-related mortality was seen in 6% of cases.
Response-adopted therapy based on interim position emission tomography (PET) is a standardized approach in HIV-negative HL. The interpretation of PET in the HIV setting is known to be challenging because of higher likelihood of false-positive findings. Preliminary data suggest that a negative interim PET may be predictive of higher PFS in patients with HIV-HL, but this needs confirmation.99
Patients with relapsed/refractory HL on effective cART should be treated with salvage chemotherapy followed by ASCT.80,81 New combination therapy including the CD30-direct immunoconjugate brentuximab vedotin and AVD (adriamycin, vinblastine, dacarbazine) are under investigation in patients with HIV-HL (Table 7).100 Immune checkpoint blockades using anti–programmed death-1 (PD-1) antibodies, nivolumab and pembrulizumab, have shown remarkable activity in the HIV-negative relapsed/refractory HL. To date, only 1 phase 1 study101 (Table 7) and case reports are reported in HIV-positive patients, because they are excluded from all clinical trials of the general population.
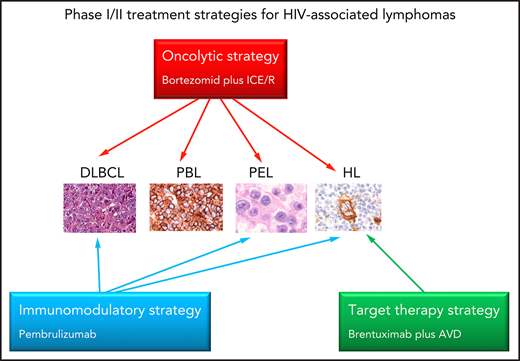
New treatment strategies for HIV-associated lymphomas
| Drug . | Study . | Mechanisms of action . | Disease . | Major results . |
|---|---|---|---|---|
| Oncolytic strategy | ||||
| Bortezomid (proteasome and HDAC inhibitor) plus ICE/R | Phase 1 | Potent oncolytic activity, cytotoxicity, inductor of apoptosis, synergistic/additive activity with CT, AZT | Relapsed/refractory DLBCL, PBL, PEL, HL | CR 77% 1-y OS 57% |
| Rapamycin (mTOR inihibitor) | Preclinical | Oncolytic activity, cytostatic activity | GHV-related malignancies | Increase expression of Zta and Rta, inihibition of mTOR Complex 1 |
| PEP005 (ingenol-3-angelate) plus JQ1 (BET inhibitor) | Preclinical | “Shock and Kill” strategy PEP005:oncolytic activity, inductor of apoptosis, inductor of NF-xB signaling JQ1: histone modification, inhibition of NF-κB signaling | PEL | Inhibition of PEL growth in vitro, delay of PEL growth in PEL xenograft tumor model. |
| Targeted therapy strategy | ||||
| Brentuximab vedotin (anti-CD30 monoclonal antibody plus microtubule-disrupting agent, MMAE) plus AVD | Phase 1/2 | Cytotoxicity, arrest cell cycle inductor apoptosis | HL | CR 100% 2-y PFS 100% with manageable toxicity |
| MLN0128 (mTOR inhibitor) | Preclinical | Cytotoxicity, inductor of apoptosis | Chemoresistant or rapamycin-resistant PEL | Inhibition of mTOR complex 1 and 2 in vitro and mouse model |
| Immunomodulatory strategy | ||||
| Pembrulizumab (monoclonal antibody-anti-PD-1) | Phase 1 | Inhibition of PD-1/PD-L1 axis | HL, PEL, DLBCL | Clinical benefit, with acceptable toxicity. Alert for polyclonal KSHV-associated B-cell proliferation |
| Lenalidomide, pomalidomide (thalidomide analogs) | Preclinical | Inhibition of MHC-I downregulation, inductors of ICAM-1 and B7-2 expression, inhibition of NF-κB signaling, antiangiogenesis | KSHV-related malignancies | Prevention of immune evasion, anti-proliferative effects |
| Daratumumab (anti-CD38 monoclonal antibody) | Preclinical case report | Cytotoxicity, elimination of CD38-positive regulatory cells (Tregs, Bregs, NK cells, and MDSCs) | KSHV-related malignancies | Antiproliferative effects, Immune-modulatory activities |
| Drug . | Study . | Mechanisms of action . | Disease . | Major results . |
|---|---|---|---|---|
| Oncolytic strategy | ||||
| Bortezomid (proteasome and HDAC inhibitor) plus ICE/R | Phase 1 | Potent oncolytic activity, cytotoxicity, inductor of apoptosis, synergistic/additive activity with CT, AZT | Relapsed/refractory DLBCL, PBL, PEL, HL | CR 77% 1-y OS 57% |
| Rapamycin (mTOR inihibitor) | Preclinical | Oncolytic activity, cytostatic activity | GHV-related malignancies | Increase expression of Zta and Rta, inihibition of mTOR Complex 1 |
| PEP005 (ingenol-3-angelate) plus JQ1 (BET inhibitor) | Preclinical | “Shock and Kill” strategy PEP005:oncolytic activity, inductor of apoptosis, inductor of NF-xB signaling JQ1: histone modification, inhibition of NF-κB signaling | PEL | Inhibition of PEL growth in vitro, delay of PEL growth in PEL xenograft tumor model. |
| Targeted therapy strategy | ||||
| Brentuximab vedotin (anti-CD30 monoclonal antibody plus microtubule-disrupting agent, MMAE) plus AVD | Phase 1/2 | Cytotoxicity, arrest cell cycle inductor apoptosis | HL | CR 100% 2-y PFS 100% with manageable toxicity |
| MLN0128 (mTOR inhibitor) | Preclinical | Cytotoxicity, inductor of apoptosis | Chemoresistant or rapamycin-resistant PEL | Inhibition of mTOR complex 1 and 2 in vitro and mouse model |
| Immunomodulatory strategy | ||||
| Pembrulizumab (monoclonal antibody-anti-PD-1) | Phase 1 | Inhibition of PD-1/PD-L1 axis | HL, PEL, DLBCL | Clinical benefit, with acceptable toxicity. Alert for polyclonal KSHV-associated B-cell proliferation |
| Lenalidomide, pomalidomide (thalidomide analogs) | Preclinical | Inhibition of MHC-I downregulation, inductors of ICAM-1 and B7-2 expression, inhibition of NF-κB signaling, antiangiogenesis | KSHV-related malignancies | Prevention of immune evasion, anti-proliferative effects |
| Daratumumab (anti-CD38 monoclonal antibody) | Preclinical case report | Cytotoxicity, elimination of CD38-positive regulatory cells (Tregs, Bregs, NK cells, and MDSCs) | KSHV-related malignancies | Antiproliferative effects, Immune-modulatory activities |
AVD, doxorubicin, vinblastine, dacarbazine; BET, bromodomain extra terminal protein; Bregs, regulatory B cells; CT, chemotherapy; GHV, gammaherpesvirus; HDAC, histone deacetylases; ICAM-1, intercellular cell adhesion molecule-1;ICE/R, ifosfamide, carboplatin, etoposide/rituximab; MDSCs, myeloid-derived suppressor cells; MHC-1, major histocompatibility complex-1; MMAE, monomethyl auristatin; mTOR, mammalian target rapamycin; NF-κB, nuclear factor κB; NK, natural killer; PD-L1, programmed death ligand 1; Tregs, regulatory T cells; zta and rta, 2 immediate-early transcription factors that regulate the expression of key EBV lytic genes.
Emerging clinical issues related to KSHV-associated MCD
The incidence of KSHV-MCD (Figure 7) has increased among HIV-infected patients on cART and is almost certainly underestimated because the symptoms are not specific.102,103 A degree of immunocompetence and immune dysregulation is necessary to develop this remitting-relapsing disease. KSHV viral load and serum cytokines correlate with systemic symptoms, effusions, and laboratory abnormalities associated with active disease (ie, cytopenias, hypoalbuminemia, hyponatremia, hypocholesterolemia, and elevated inflammatory markers). Concurrent KS is present in 52% to 72% of patients at diagnosis, and most progress to PEL or large B-cell lymphoma during the clinical course.37,103,104 There is no single consensus definition of MCD flare and no consensus guidelines for treatment response criteria.37
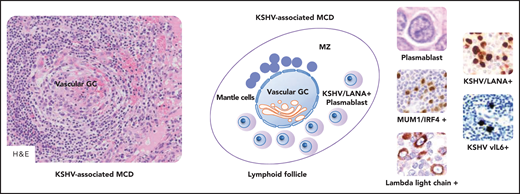
KSHV-associated MCD. KSHV-positive plasmablasts express monotypic λ light chain, IgM, CD19, and MYC; plasmablasts are also positive for CD38, CD45, and CD79a and are usually negative for CD10, CD20, CD30, CD138, BCL6, PAX5, T-cell antigens, and EBV infection (data not shown). A schematic lymphoid follicle in KSHV-associated MCD is depicted at the center. The follicle mantle zone (MZ) is typically rich in small mantle lymphocytes that are mixed with plasmablasts. These latter cells are also found at the boundary between the GC and the MZ. Vascular transformation of the GC is also depicted. Vascular GC is observed in KSHV-associated MCD as shown in the microphotograph placed on the left in the figure. As shown on the right in the figure, large cells with plasmablastic features are positive for MUM1/IRF4, λ light chain, ORF73/LANA encoded by KSHV, and viral IL6. MUM1, λ light chain, ORF73/LANA, v-IL6, immunohistochemistry, hematoxylin counterstain. Original magnification, ×200 (H&E on the left), ×1000 (H&E on the left), and ×400 (other images). Images were taken using a Nikon Eclipse 80i microscope with Plan Fluor 20×/0.75, Plan Fluor 40×/0.75, and Plan Apo/1.40 oil objectives and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
KSHV-associated MCD. KSHV-positive plasmablasts express monotypic λ light chain, IgM, CD19, and MYC; plasmablasts are also positive for CD38, CD45, and CD79a and are usually negative for CD10, CD20, CD30, CD138, BCL6, PAX5, T-cell antigens, and EBV infection (data not shown). A schematic lymphoid follicle in KSHV-associated MCD is depicted at the center. The follicle mantle zone (MZ) is typically rich in small mantle lymphocytes that are mixed with plasmablasts. These latter cells are also found at the boundary between the GC and the MZ. Vascular transformation of the GC is also depicted. Vascular GC is observed in KSHV-associated MCD as shown in the microphotograph placed on the left in the figure. As shown on the right in the figure, large cells with plasmablastic features are positive for MUM1/IRF4, λ light chain, ORF73/LANA encoded by KSHV, and viral IL6. MUM1, λ light chain, ORF73/LANA, v-IL6, immunohistochemistry, hematoxylin counterstain. Original magnification, ×200 (H&E on the left), ×1000 (H&E on the left), and ×400 (other images). Images were taken using a Nikon Eclipse 80i microscope with Plan Fluor 20×/0.75, Plan Fluor 40×/0.75, and Plan Apo/1.40 oil objectives and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2. Images were processed using Adobe Photoshop CS2 V9.0.
Untreated KSHV-MCD is usually fatal, with the occurrence of hemophagocytic syndrome, multiple organ failure, infections, or transformation to lymphoma.37,104 There are no approved treatment approaches. However, prospective trials support the use of rituximab given alone or in combination with chemotherapy.63,105,106 Rituximab monotherapy, reducing autocrine and paracrine signaling in tissue microenvironment, resulted in a 5-year OS rate of 90% and was associated with an 11-fold lower risk of developing lymphoma.106 A common problem (35% to 67% of cases) was exacerbation of KS.105,106 For patients with concurrent/worsening KS or multiple organ failure, the combination of rituximab and liposomal doxorubycin resulted in 3-year OS of 81% and EFS of 69%.63 Multimodality therapies focusing on HIV and KSHV replication, proliferation of KSHV-infected and -uninfected B cells, endothelial cells, and cytokine signaling are urgently needed.
Preventive measures
Early cART access and maintenance of immune recovery during HIV infection remain key strategy for prevention of infection-related malignancies, including lymphoma.107 This benefit is attributable to HIV suppression, CD4 cell recovery, and other mechanisms impacting coinfections with oncogenic viruses (ie, CD8 response recovery and reduction of inflammation).108,109
The survival of many people living with HIV and cancer is now approaching that of the general population. Long-term surveillance programs should be performed because cancer survivors are at increased risk for subsequent primary cancers, because of the persistence of the etiologic agents and the immunosuppressive/carcinogenic effects of treatments.110,111 A population-based linkage study found that 9% of all incident primary HIV-associated cancers were second or later cancers in the United States, a proportion similar but with higher incidence compared with that in the general population. From 1990 to 2010, SIR for second primary cancers was elevated for KS (28.0), anal cancer (17.0), NHL (11.1), HL (5.4), and liver cancer (3.6). First and second primary AIDS-defining cancers decreased and second non–AIDS-defining cancers increased over time.110 Recently, another large linkage study (1996-2015) found an increased risk for second primary nonlymphoid cancers following a lymphoid malignancy, specifically for myeloid malignancies, KS, and human papilloma virus (HPV)-associated cancers, including anal, vaginal/vulvar, and rectal squamous cell cancer.111 Disease preventive measures, such as immunization (HPV and HBV vaccination), and early disease detection through screening programs18 for secondary primary cancers are recommended.
Currently, we are facing the global public health crisis of the SARS CoV-2 and COVID-19 pandemic. Older age and comorbidities including cancer are risk factors for severe disease and death, with the case fatality rate higher for patients with hematologic malignancies. The effect of HIV coinfection on the clinical course of COVID-19 has not yet been fully elucidated; however, HIV-infected patients with hematologic cancers may be at greater risk for severe disease. No proven effective therapies currently exist for COVID-19. Infection prevention and control, close monitoring during anticancer therapy, and persistent HIV care are mandatory.
Therapeutic perspectives
The current classification and the diagnostic workup of HIV-associated lymphomas are considerably improving the choice of treatment, but the management of these patients is still challenging. Treatment management remains challenging in patients with PEL, PBL, and in those with high IPI score NHL. Research priorities are pathogenesis-direct treatment strategies including oncolytic therapies,112 targeted therapies for EBV or KSHV-associated lymphomas,100 and immunomodulatory approaches101,113 (Table 7). Oncolytic strategy is expected to induce oncolysis by viral replication and immune-mediated cell death of cancer cells latently infected by EBV-KSHV.112 PEL are addicted to the mammalian target of rapamycin (mTOR) signaling pathway. Thus, targeted therapy with mTOR inhibitors such as rapamycin and MNL0128 (sapanisertib) may represent a promising new treatment approach for these patients.114 Immunotherapy with checkpoint inhibitors has demonstrated significant activity in a broad range of cancers of the general population, including HL. A phase 1 study with pembrolizumab strongly supports the use of anti–PD-1 monoclonal antibody therapy in HIV-infected patients with cancer on cART and with CD4 count ≥ 100/µL.101 The antiangiogenetic drugs lenalidomide and pomalidomide have shown immunomodulatory activity in vitro, including prevention of KSHV-induced immune evasion. Daratumumab, an anti-CD38 human monoclonal antibody, has the potential to be an effective treatment for patients with CD38-positive lymphoma including PEL,113 targeting CD38-positive tumor cells and immunosuppressive regulatory cells of tumor microenvironment.115
A recent randomized phase 2 study demonstrated that adding the oncolytic vorinostat to EPOCH (plus rituximab if CD20 positive) had no effect on CR and EFS rates or on HIV reservoir size in aggressive NHL. Only MYC expression was a biomarker of poor prognosis, suggesting that novel drugs targeting oncogenic alterations are urgently needed in these patients.73
To date, most cancer clinical trials exclude all HIV-infected patients. The HIV Working Group has defined important principles related to eligibility criteria for HIV-infected patients with cancer. Notably, patients with CD4 count ≥350/mL should generally be eligible for any study, whereas lower CD4 count may be appropriate in the setting of second-line and later cancer therapy, assuming cART is carefully managed. All these studies will require multidisciplinary efforts.
Acknowledgments
A.C. is a member of World Health Organization International Agency for Research on Cancer Monograph Working Group on Biological Agents, Lyon 2019. This work was supported, in part, by an Institutional grant from Centro di Riferimento Oncologico, Aviano for an Intramural Project “Agenti infettivi e Tumori” (A.C., E.V.), and the Italian Ministry of Health (5 × 1000 Funds–2015), through Institutional Grant BRI2018 “Progetto 6—Visualizing immunomodulatory molecules in individual human cancer cells using in situ bright field multiplexing methods and an innovative proximity detection assay” (A.G.).
Authorship
Contribution: A.C. designed the review and overviewed the manuscript; and all authors wrote the manuscript, reviewed the article before submission, and approved the final version after revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonino Carbone, Centro di Riferimento Oncologico, IRCCS, National Cancer Institute, Via Franco Gallini 2, I-33081 Aviano (PN), Italy; e-mail: acarbone@cro.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal