In this issue of Blood, Zoref-Lorenz et al report the development of an optimized hemophagocytic lymphohistiocytosis (HLH) inflammatory index (OHI) that discriminates paraneoplastic inflammation in hematologic malignancies from pathologic hyperinflammation, known as malignancy-associated HLH.1
A patient with remittent fever, mild or missing lymphadenopathy, splenomegaly, and progressing cytopenia who is at risk of developing respiratory failure, hepatitis, encephalopathy, and coagulopathy may be experiencing severe infection, sepsis, or systemic inflammatory response syndrome, or he or she may have HLH. Depending on the awareness level, one may suspect HLH early during the diagnostic workup. But when HLH is suspected, management becomes complicated. When and how should treatment be started? What could be the underlying trigger? How should unspecific pathology reports be dealt with? Is it really HLH or simply a condition that mimics HLH? Are high-risk invasive diagnostic procedures like biopsies of liver, spleen, or lymph node justified in a patient with multiorgan dysfunction syndrome and coagulopathy?
Zoref-Lorenz et al investigated an international multicenter cohort of 225 patients with hematologic malignancies. Adult patients with confirmed HLH according to the HLH-2004 diagnostic criteria, which were developed for familial HLH in children,2 were compared with patients with hematologic malignancies without paraneoplastic HLH. The authors wished to better define dangerous paraneoplastic hyperinflammation, which is a diagnostic fogbank that delays the identification of the triggering malignancy3 and a prognostic disaster for patients with lymphoma.4 Paraneoplastic HLH affects ∼1% of adult patients with hematologic malignancies and up to 3% of those with lymphomas. For these patients, the uncertainty regarding the diagnostic validity of the pediatric HLH-2004 criteria has been a deadly knowledge gap causing toxic, nonrational, or ineffective treatment.5 Therefore, the OHI has the potential to significantly reduce morbidity and mortality resulting from late or failed diagnosis. Key to this innovation as proposed in the clinical routine is its simplicity. The authors defined threshold values for the 2 key HLH-2004 serum markers, ferritin (>1000 ng/mL) and soluble CD25 (sCD25; >3900 U/mL,) to indicate pathologic and prognostically relevant hyperinflammation in adult patients with confirmed malignancy-associated HLH. In children, who are rarely affected by lymphoma, the HLH-2004 diagnostic thresholds are defined as >500 ng/mL for ferritin and >2400 U/mL for sCD25.2 The uncertainty regarding age- and disease-dependent selection of relevant diagnostic parameters and their respective threshold values has led to the proposal of alternative scores that either lack validation in hematology patients (HScore)6 or require complex addition of a multitude of parameters.7 The HLH lead triad of fever, cytopenia, and splenomegaly combined with hyperferritinemia as a diagnostic red flag overlaps the diagnostic findings in lymphoma, myelodysplastic syndrome, and other myeloid or lymphoid malignancies and is therefore nonspecific. Some have called HLH “a faith-based diagnosis, making the phenotype of the provider as important as the patient in identifying and reporting HLH, versus other conditions characterized by inflammation.”8(p181) The OHI helps to establish HLH as dangerous hyperinflammation early in the workup of our patients, independent of our so-called provider phenotype. Multiple analyses comparing HLH 2004 criteria vs the OHI, the HScore vs the OHI, the OHI in treatment-naïve patients vs in treatment-conditioned patients, checking the OHI at presentation vs checking the OHI with peak OHI parameters, or centers with routine ferritin/sCD25 screening vs those with assessment by HLH suspicion now provide us with some important novel insights. Patients with lymphoid or myeloid neoplasms, OHI− at presentation, have an excellent prognosis. OHI+ patients are depicted by terrifying Kaplan-Meier curves. This calls for action. Disease-specific prognostication can be optimized by including the OHI in prospective protocols. The authors have already taken the initiative to prospectively apply this approach in their institutions, and the results of this registry are eagerly awaited. The OHI, however, also allows for prospective treatment-stratification trials that integrate anti-inflammatory agents such as etoposide or cytokine-directed treatment against interleukin-1 receptor, interleukin-6, or interferon-γ or small-molecule kinase inhibitors directed against Janus kinases in combined disease-specific immunochemotherapy protocols.9
It is important to note that HLH in malignancy presents as paraneoplastic hyperinflammation, which leads to a workup searching for the underlying malignancy. Acquired HLH, however, also is seen in patients receiving treatment for malignancy, when infections trigger aberrant hyperinflammation.10 After having received numerous transfusions leading to hyperferritinemia, presenting in chemotherapy-induced aplasia with disease-inherent splenomegaly, patients have additional overlapping diagnostic features, making it difficult to discriminate fever resulting from infection vs fever resulting from infection-triggered HLH requiring immunomodulation. Such patients were excluded from the OHI cohort and therefore represent an important clinical scenario for future research. More urgent questions can now be addressed by applying extended lymphoma characterization using the terms OHI+ vs OHI−. Would T cell–engaging therapies (eg, bispecific antibodies, bispecific T-cell engagers, or chimeric antigen receptor T cells) in patients with OHI+ lymphoma pose an extra risk in terms of excess toxicity through iatrogenic cytokine release syndrome requiring preemptive cytokine-directed treatment? Would patients with OHI+ Hodgkin lymphoma be prone to developing extra immune-related toxicity when treated with checkpoint inhibitors? Would patients with OHI+ lymphoma benefit from etoposide as part of lymphoma-directed treatment or from tailored consolidation strategies such as primary high-dose chemotherapy with autologous stem cell transplantation?
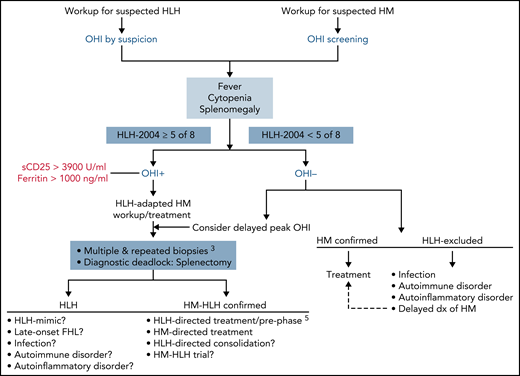
The prerequisite for broad application of the OHI is the availability and timely laboratory turnaround time of sCD25 serum levels. This was excluded from the HScore because of restricted availability.6 Furthermore, an institutional decision toward routine screening vs assessment only by suspicion could be a systemic control measure to reduce the diagnostic failure rate and (too) late application of the OHI in treatment-conditioned, inflammation-suppressed patients. These adaptations, combined with clinical experience and so-called gut feeling, which is also addressed in the calculations performed by Zoref-Lorenz et al, will reduce the time from symptom onset to diagnosis of malignancy-associated HLH (see figure for proposed OHI-adapted workup). It is also hoped they will increase the detection rate of hematologic malignancies hiding behind the diagnostic fogbank that is HLH. Ultimately, timely diagnosis of the underlying condition will allow disease-specific treatment and prevent excess mortality in patients classified as having HLH of unknown trigger.
OHI-adapted HLH and hematologic malignancy (HM) workup. Early ordering of serum sCD25 and ferritin directs further testing and allows for adequate HLH control in OHI+ patients by concomitant immunosuppression before onset of end-organ damage.5 It also affects selection of diagnostic procedures aimed at exposing occult malignancies through adapted invasive multiorgan biopsies.3 dx, diagnosis; FHL, familial HLH.
OHI-adapted HLH and hematologic malignancy (HM) workup. Early ordering of serum sCD25 and ferritin directs further testing and allows for adequate HLH control in OHI+ patients by concomitant immunosuppression before onset of end-organ damage.5 It also affects selection of diagnostic procedures aimed at exposing occult malignancies through adapted invasive multiorgan biopsies.3 dx, diagnosis; FHL, familial HLH.
Conflict-of-interest disclosure: P.L.R. has received honoraria from Sobi (Adboard) and Novartis (Adboard).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal