In this issue of Blood, Pecker et al1 report that some women with sickle cell anemia (HbSS) have diminished ovarian reserve and argue for equitable access to fertility preservation strategies, which are offered as standard care to women with cancer.
Early reports documented the profound effects that sickle cell disease has on normal sexual development. In the landmark Jamaican natural history study, for example, females with HbSS had delayed sexual development, with menarche occurring over 2 years later than controls.2 Despite these initial descriptions, the effects of sickle cell disease and its treatments on fertility in women have received surprisingly little attention. This is a topical and important issue because most females in high-resource settings survive into their reproductive years and are in better overall health because of disease-modifying therapies like hydroxyurea.
In the few published studies, there appears to be an acceleration of the age-associated decline in ovarian reserve in women with sickle cell disease, some of whom develop diminished ovarian reserve (DOR), or low egg supply, which is a known risk factor for infertility. In one US, single-institution, cross-sectional study, 56 females with HbSS and reproductive potential (average age 14 years) had assessments of ovarian reserve using the proxies serum anti-Müllerian hormone (AMH) and follicle stimulating hormone. DOR was documented in 30% of these young patients, most commonly in those who previously received stem cell transplantation.3 Similarly, a United Kingdom (UK)-based, single-institution study of 50 women with sickle cell disease (mostly HbSS, average age 35 years) demonstrated that 55% had either negligible or reduced serum AMH levels consistent with DOR.4 More recently, a retrospective analysis of 93 young women with HbSS (average age 30 years) enrolled in the Multicenter Study of Hydroxyurea (MSH) documented premature DOR in 75%.5
Pecker et al assessed ovarian reserve in 26 young women with HbSS, between 19 and 30 years of age, by AMH measurements, which was supplemented by direct antral follicle counts by ultrasonography in 19 study participants (the COVID-19 pandemic prevented this planned testing in all 26). Although this is a small sample, it adds meaningfully to the limited body of published data on this topic. The investigators found an expected age-associated decline in AMH, but most patients (21/26, 80%) did not have DOR, which is encouraging.
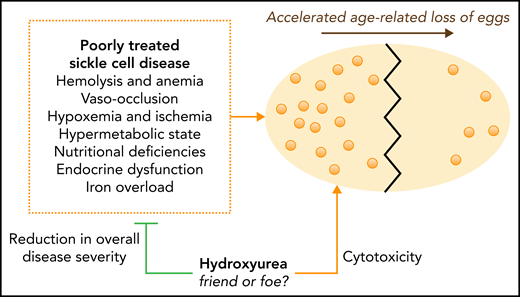
See the figure for an illustration of the potential causes of early or accelerated DOR in sickle cell disease. In the Jamaican cohort, a multifactorial etiology was proposed, including abnormal endocrine function due to suboptimal nutrition and a hypermetabolic state.6 Others have proposed iron overload as a risk factor or a direct effect of recurrent ovarian sickling and vaso-occlusion leading to tissue ischemia and organ infarction.7 The recent observation that prepubertal males with HbSS have reduced spermatogonial stem cells, compared with controls, is consistent with insidious gonadal tissue damage due to chronic sickling with consequences that manifest in young adults.8
What about the potential role of hydroxyurea in increasing the risk for DOR? In the previously noted US study,3 low AMH levels were found in 24% of teenagers on hydroxyurea but were not seen in untreated patients. In the UK study,4 few women were prescribed hydroxyurea, so no conclusions could be reached. In the retrospective MSH analysis,5 all of the women were receiving hydroxyurea at the time of sample collection. There was a strong selection bias for clinical severity, however, because frequent vaso-occlusive events were necessary to be eligible for treatment. In the current study,1 the 5 women with DOR were currently taking hydroxyurea, whereas 10 of 21 without DOR were currently taking hydroxyurea. Notably, 19 of 21 without DOR had taken hydroxyurea at some point (current or past use). Converting these numbers into standard epidemiological terms, the number needed to harm (NNH) is 3.0 (95% CI, 1.7-10.6) or 4.8 (2.7-21.8), considering current or any use of hydroxyurea, respectively. Stated plainly, ∼3 to 5 women would need to be prescribed hydroxyurea for DOR to be found in 1, depending on the definitions used.
It is important to remember that this NNH statistic does not tell us what is actually causing the harm (DOR). As a cytotoxic agent, hydroxyurea is often blamed for myriad deleterious effects including infections, poor growth, infertility, teratogenicity, mutagenicity, and carcinogenicity, even though real-life experiences do not support these concerns in sickle cell disease. In addition, hydroxyurea has historically been prescribed primarily to the most severely affected patients. This makes it difficult to know whether hydroxyurea is a proxy for more severe underlying disease with vaso-occlusive organ damage or hydroxyurea at least partly causes DOR. Theoretically, by improving anemia and reducing vaso-occlusive complications, hydroxyurea could protect ovarian tissue and egg reserve (see figure). Longitudinal follow-up of pediatric cohorts treated with hydroxyurea early in life are needed to help quantify the risk of DOR and, especially, to assess the actual risk of infertility. In MSH, there were over 100 children born to hydroxyurea-treated patients,9 and a recent “real-life” European cohort of 643 adult females receiving hydroxyurea also documented over 100 pregnancies.10
Accelerated age-related decline in ovarian reserve in sickle cell disease. An ovary is shown (large, yellow oval) undergoing an accelerated loss of eggs (small, light-orange circles) over time. Untreated or poorly-treated sickle cell disease damages multiple tissues and organs by multiple mechanisms. Disease-related, direct ovarian damage could lead to an accelerated loss of egg supply and diminished ovarian reserve. Hydroxyurea decreases clinical severity and organ damage in sickle cell disease, potentially preserving ovarian reserve. Theoretically, the potential cytotoxic effects of hydroxyurea on ovarian reserve could partially offset this benefit. Assessment of ovarian reserve can allow early and equitable discussions about fertility preservation.
Accelerated age-related decline in ovarian reserve in sickle cell disease. An ovary is shown (large, yellow oval) undergoing an accelerated loss of eggs (small, light-orange circles) over time. Untreated or poorly-treated sickle cell disease damages multiple tissues and organs by multiple mechanisms. Disease-related, direct ovarian damage could lead to an accelerated loss of egg supply and diminished ovarian reserve. Hydroxyurea decreases clinical severity and organ damage in sickle cell disease, potentially preserving ovarian reserve. Theoretically, the potential cytotoxic effects of hydroxyurea on ovarian reserve could partially offset this benefit. Assessment of ovarian reserve can allow early and equitable discussions about fertility preservation.
Although uncertainties remain, Pecker et al do not argue to limit the use of hydroxyurea, because it is a transformative therapy that improves and prolongs life. Instead, they argue against the false choice between the preservation of fertility and use of hydroxyurea. Women with sickle cell disease can have both. Regardless of the potential risks or benefits of hydroxyurea for ovarian reserve, shouldn’t we offer the same range of fertility counseling and preservation strategies offered to other women with risk of DOR and infertility, such as those with cancer? This reproductive inequity is one example of many faced by individuals with sickle cell disease, a population that remains marginalized and neglected. We must consider the role of systemic racism in health care as we seek to eliminate this particular disparity. Although we can be encouraged that most females with sickle cell disease do not develop early DOR, fertility services should be provided as standard clinical care for girls and women in conjunction with ongoing, optimized disease-modifying therapy.
Conflict-of-interest disclosure: C.T.Q. declares no competing interests. R.E.W. receives research donation of hydroxyurea from Bristol Myers Squibb and Addmedica, and is a medical advisor for Nova Laboratories.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal