Key Points
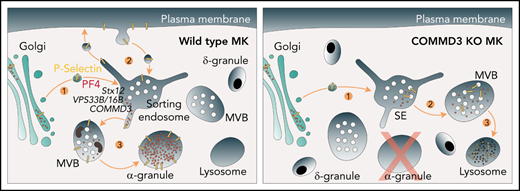
Stx12 and the COMMD3/CCC complex interact physically and functionally with disease-associated proteins VPS33B and VPS16B inf α-granule biogenesis.
Megakaryocytes repurpose the ubiquitous endosome retrieval and recycling machinery for trafficking of α-granule proteins.
Abstract
Platelet α-granules regulate hemostasis and myriad other physiological processes, but their biogenesis is unclear. Mutations in only 3 proteins are known to cause α-granule defects and bleeding disorders in humans. Two such proteins, VPS16B and VPS33B, form a complex mediating transport of newly synthesized α-granule proteins through megakaryocyte (MK) endosomal compartments. It is unclear how the VPS16B/VPS33B complex accomplishes this function. Here we report VPS16B/VPS33B associates physically with Syntaxin 12 (Stx12), a SNARE protein that mediates vesicle fusion at endosomes. Importantly, Stx12-deficient MKs display reduced α-granule numbers and overall levels of α-granule proteins, thus revealing Stx12 as a new component of the α-granule biogenesis machinery. VPS16B/VPS33B also binds CCDC22, a component of the CCC complex working at endosome exit sites. CCDC22 competes with Stx12 for binding to VPS16B/VPS33B, suggesting a possible hand-off mechanism. Moreover, the major CCC form expressed in MKs contains COMMD3, one of 10 COMMD proteins. Deficiency of COMMD3/CCDC22 causes reduced α-granule numbers and overall levels of α-granule proteins, establishing the COMMD3/CCC complex as a new factor in α-granule biogenesis. Furthermore, P-selectin traffics through the cell surface in a COMMD3-dependent manner and depletion of COMMD3 results in lysosomal degradation of P-selectin and PF4. Stx12 and COMMD3/CCC deficiency cause less severe phenotypes than VPS16B/VPS33B deficiency, suggesting Stx12 and COMMD3/CCC assist but are less important than VPS16B/VPS33B in α-granule biogenesis. Mechanistically, our results suggest VPS16B/VPS33B coordinates the endosomal entry and exit of α-granule proteins by linking the fusogenic machinery with a ubiquitous endosomal retrieval complex that is repurposed in MKs to make α-granules.
Introduction
Platelet α-granules are formed in the platelet progenitor, the megakaryocyte (MK).1-5 α-granules are lysosome-related organelles6 loaded with hundreds of proteins that are either synthesized by the MK or internalized via endocytosis.5,7-10 Remarkably, VPS16B and VPS33B are among a few documented proteins that, when mutated, disrupt α-granule biogenesis.4,11-14 VPS16B and VPS33B form a stable complex, and either of them is mutated in patients with arthrogryposis, renal dysfunction, and cholestasis syndrome13,14 who, among other manifestations, present with coagulation deficiencies due to aberrant α-granule formation. Understanding α-granules in health and disease will require identifying the cellular mechanism and molecular components responsible for their biogenesis.
In previous work,15 we showed that newly synthesized α-granule proteins are transported through endosomes on their way to α-granules and that the VPS16B/VPS33B complex localizes to endosomes. Furthermore, lack of VPS33B targeted α-granule proteins for degradation in lysosomes.15 Based on sequence homology, VPS33B belongs to the Sec1/Munc18 (SM) family of proteins that regulate SNARE protein-mediated vesicle fusion.14 However, it is unknown if the VPS16B/VPS33B complex indeed interacts with SNARE proteins and, if so, which of the 38 human SNARE proteins may be involved.
A complex known as CCC (COMMD/CCDC22/CCDC93) is emerging as a fundamental regulator of protein cargo traffic through endosomes in many cell types.16-18 A recent report showed the VPS16B/VPS33B complex interacts physically with the CCC complex in nonspecialized cells via the CCDC22 subunit.19 Moreover, there are 10 members in the COMMD family generating distinct forms of the CCC complex, and although they are all widely expressed in human tissues, the relative level of expression of individual COMMDs differs among cell types,20 and diverse phenotypes have been observed when different COMMDs have been manipulated.21-23 It is unknown if the CCC complex functions in α-granule biogenesis and which COMMD may have a preponderant role.
In various cell types, the CCC complex works at endosome exit sites together with other factors and the cargo adaptor protein SNX17 that recognizes integral membrane protein cargo containing a specific sorting signal in their cytosolic domain.18,24,25 This machinery facilitates endosomal traffic of SNX17 cargo by retrieving them from lysosomal degradation and promoting their traffic to other cellular destinations such as the cell surface.24 Interestingly, some platelet α-granule proteins like P-selectin contain sorting signals bound by SNX17,25 and their intracellular traffic depends on SNX17 in other cells.26-28 However, at steady state, P-selectin is not present on the cell surface of platelets/megakaryocytes, and it is unknown if SNX17 participates in traffic to α-granules.
Here, we show that VPS16B/VPS33B interacts specifically with the SNARE protein Syntaxin 12 (Stx12) and that deficiency of Stx12 results in defective α-granule biogenesis, thus defining Stx12 as a new machinery component. We established the CCC complex is needed for α-granule biogenesis and that COMMD3 defines a major CCC form at discrete endosome locations in cells actively making α-granules. We found that in megakaryocytes, P-selectin traffics through the cell surface in a COMMD3- and SNX17-dependent manner and that COMMD3 is critical for retrieval of both α-granule soluble and transmembrane proteins from a degradative fate.
Methods
Cell culture, transfection, and generation of CRISPR knockout (KO) imMKCL cells
imMKCL cells were a gift from Koji Eto (Kyoto University) and were cultured, differentiated, and transfected as described.29 See supplemental Methods for more details and information on CRISPR KO imMKCL cells generation.
DNA constructs and biochemical procedures
All DNA constructs and mutations were generated using the In-Fusion HD Cloning Plus kit (Takara) and corroborated by sequencing. Recombinant fusion proteins 6-His-VPS33B (His-VPS33B) and Twin Strep-VPS16B cloned into a bicistronic pET-Duet plasmid were expressed in BL21 codon plus Escherichia coli and first purified using the TALON cobalt affinity resin (Thermo Scientific) as described previously15,30,31 except for the elution that was carried out with 10 mM EDTA. The eluate was then incubated with MagStrep “type 3” XT beads (iba), and magnetic beads were washed and eluted following manufacturer’s recommendations. GST-protein purification and pulldowns were done as described.32 For the Twin Strep pulldowns 6-His-VPS33B (His-VPS33B) and Twin Strep-VPS16B were purified as before but were not eluted from the MagStrep “type 3” XT beads (VV beads). The VV beads were incubated with purified GST or His proteins in phosphate buffer saline pH 7.4 supplemented with 50 mM NaCl and 0.2% TX-100 for 1 hour at room temperature with rotation. Subsequently, the VV beads were washed 3 times with the same buffer and boiled 5 minutes in 1x sample buffer. Immunoblotting was performed as previously described.33 Additional information can be found under supplemental Methods.
Fluorescence and electron microscopy
Spinning disk confocal images were obtained as previously described34-36 using a temperature-controlled chamber at 37°C and 5% (vol/vol) CO2 on an Olympus IX81 spinning disk confocal microscope with Photometrics Cascade II camera and a 100x/1.40NA objective (3i). Excitation was performed with diode lasers of 405 nm (BFP2), 488 nm (GFP, Alexa Fluor 488), 561 nm (Cherry), and 640 nm (Alexa Fluor 647). The Slidebook software (3i) controlled all aspects of acquisition and analysis. Image analysis is described in supplemental Methods. Superresolution images (resolution = 120 nm) were obtained using a Zeiss LSM 900 laser scanning confocal microscope with a Plan-Apochromat 63x/1.40 Oil DIC M27 objective and the ZEN Blue software in Super Resolution mode. Raw images were processed using the Zen Blue software’s Airyscan Processing function. In addition to Figure 6E, we present other examples of super-resolution images of COMMD3 KO imMKCL cells transfected with GFP-COMMD3 and Cherry-Stx12 illustrating the subendosomal localization of COMMD3 on Stx12 structures as supplemental Figure 16. For electron microscopy, pelleted cells were high-pressure frozen using a Wohlwend Compact 02 high-pressure freezer (Technotrade International, Manchester, NH) as described37 and imaged with a Tecnai T12 Spirit TEM, operating at 100 kV using an AMT CCD camera. See supplemental Methods for additional information.
RNA-seq
Four RNA-independent samples from both undifferentiated and differentiated imMKCL were submitted for RNAseq analysis to Novogene. See supplemental Methods for details.
Results
The VPS16B/VPS33B complex binds to the Qa-SNARE protein Stx12
SNARE proteins are classified into 4 subfamilies, Qa-, Qb-, Qc-, and R-SNAREs. Current models posit that SM proteins first bind to a particular Qa-SNARE (Stx), which typically resides in the membrane of the target compartment.38 To test for the possibility that the VPS16B/VPS33B complex binds a Qa-SNARE, we cloned and purified the cytosolic domain of all 13 human Qa-SNAREs as GST-fusions. We also purified recombinant VPS16B/VPS33B complex.15 GST-pulldown assays with the GST-Qa-SNAREs revealed an interaction between the VPS16B/VPS33B complex and Stx12 (Stx13), and to a lesser extent, Stx11 and Stx17 (Figure 1A). The interaction between Stx12 and VPS16B/VPS33B was confirmed by coimmunoprecipitation of the endogenous proteins using MEG-01 total cell extracts (Figure 1B). Syntaxins contain regulatory regions (Figure 1C) that may interact with the corresponding SM protein (N-peptide) or intramolecularly bind the SNARE domain (Habc), rendering the Stx protein in a close conformation.38 To test these possibilities, we performed GST-pulldown assays with various Stx12 fragments. The VPS16B/VPS33B complex bound strongly to the SNARE domain and weakly to the whole cytosolic domain while binding to the N-peptide and Habc fragments was negligible (Figure 1D-E). The data suggest the interactions with VPS16B/VPS33B are mediated by the Stx12 SNARE domain and that the N-terminal region of Stx12, likely the Habc domain, has an inhibitory effect. Phosphorylation of the Habc domain of Stx12 at Serine 139 has been shown to promote binding of Stx12 to its cognate R-, Qb-, and Qc-SNARE partners, presumably by facilitating the opening of the Stx12 conformation.39 Introduction of a phosphomimetic mutation (S139D) in the GST-Stx12 cytosolic fragment caused an increase in binding to VPS16B/VPS33B, thus supporting an inhibitory function for the Stx12 Habc domain (supplemental Figure 1). As an additional specificity control, GST-Stx12 SNARE robustly pulled down VPS16B/VPS33B while GST-Stx4 SNARE and GST-Stx5 SNARE did not (Figure 1F-G).
VPS33B binds to the SNARE domain of endosomal Stx12. (A) GST-pulldown assay. GST fusion proteins corresponding to the whole cytosolic domain of each of the 13 human Syntaxins (Qa-SNAREs) were bound to glutathione beads and incubated with purified Twin Strep-VPS16B/His-VPS33B complex. Top panel, the bound Twin Strep-VPS16B/His-VPS33B complex was detected by immunoblotting (IB) with antibodies against the His-VPS33B polyhistidine tag (His). Bottom panel, SDS-PAGE (Coomassie), indicating comparable loading amounts of the GST-fusion proteins. (B) Coimmunoprecipitation assay. A total MEG-01 cell extract was incubated with rabbit anti-Stx12 antibody bound to Sepharose beads or irrelevant rabbit IgG bound to Sepharose beads as a control. Bound proteins were analyzed by IB with antibodies against VPS33B and Stx12. (C) Cartoon depicting Stx12 domains. (D) GST-pulldown assay with the whole Stx12 cytosolic domain (Cyt) or the indicated fragments (N-Pep, Habc, SNARE) fused to GST and purified Twin Strep-VPS16B/His-VPS33B complex. The bound Twin Strep-VPS16B/His-VPS33B complex was detected by IB with antibodies to both VPS33B and VPS16B. SDS-PAGE (Coomassie) indicates comparable loading amounts of the GST-fusion proteins. (E) Quantification of the GST pulldown shown in D. The bars indicate the ratio of VPS33B pulled down by each of the Stx12 fragments over the whole cytosolic domain of Stx12 (n = 3). All samples were compared with GST alone, and statistical significance was determined via unpaired, 2-tailed Student t test. (F) GST-pulldown assay with the SNARE domains of Stx4, Stx5, and Stx12 fused to GST and purified Twin Strep-VPS16B/His-VPS33B complex. The bound Twin Strep-VPS16B/His-VPS33B complex was detected by IB with an antibody against VPS33B. SDS-PAGE (Coomassie) indicates comparable loading amounts of the GST-fusion proteins. (G) Quantification of the GST pulldown shown in F. The bars indicate the ratio of VPS33B pulled down by each of the SNARE fragments over GST (n = 3). All samples were compared with GST alone, and statistical significance was determined via unpaired, 2-tailed Student t test.
VPS33B binds to the SNARE domain of endosomal Stx12. (A) GST-pulldown assay. GST fusion proteins corresponding to the whole cytosolic domain of each of the 13 human Syntaxins (Qa-SNAREs) were bound to glutathione beads and incubated with purified Twin Strep-VPS16B/His-VPS33B complex. Top panel, the bound Twin Strep-VPS16B/His-VPS33B complex was detected by immunoblotting (IB) with antibodies against the His-VPS33B polyhistidine tag (His). Bottom panel, SDS-PAGE (Coomassie), indicating comparable loading amounts of the GST-fusion proteins. (B) Coimmunoprecipitation assay. A total MEG-01 cell extract was incubated with rabbit anti-Stx12 antibody bound to Sepharose beads or irrelevant rabbit IgG bound to Sepharose beads as a control. Bound proteins were analyzed by IB with antibodies against VPS33B and Stx12. (C) Cartoon depicting Stx12 domains. (D) GST-pulldown assay with the whole Stx12 cytosolic domain (Cyt) or the indicated fragments (N-Pep, Habc, SNARE) fused to GST and purified Twin Strep-VPS16B/His-VPS33B complex. The bound Twin Strep-VPS16B/His-VPS33B complex was detected by IB with antibodies to both VPS33B and VPS16B. SDS-PAGE (Coomassie) indicates comparable loading amounts of the GST-fusion proteins. (E) Quantification of the GST pulldown shown in D. The bars indicate the ratio of VPS33B pulled down by each of the Stx12 fragments over the whole cytosolic domain of Stx12 (n = 3). All samples were compared with GST alone, and statistical significance was determined via unpaired, 2-tailed Student t test. (F) GST-pulldown assay with the SNARE domains of Stx4, Stx5, and Stx12 fused to GST and purified Twin Strep-VPS16B/His-VPS33B complex. The bound Twin Strep-VPS16B/His-VPS33B complex was detected by IB with an antibody against VPS33B. SDS-PAGE (Coomassie) indicates comparable loading amounts of the GST-fusion proteins. (G) Quantification of the GST pulldown shown in F. The bars indicate the ratio of VPS33B pulled down by each of the SNARE fragments over GST (n = 3). All samples were compared with GST alone, and statistical significance was determined via unpaired, 2-tailed Student t test.
Stx12 localizes to sorting endosomes
Stx12 localizes to endosomal compartments in other cell types.40 In order to investigate its presence in megakaryocyte sorting endosomes, we used stem cell-derived immortalized megakaryocyte cells (imMKCL), which can be differentiated in vitro and produce α-granules and functional platelets.15,29,41,42 We coexpressed BFP2-Stx12 and Cherry-SNX17, a marker of sorting endosomes,25 in imMKCL cells, and performed live-cell confocal fluorescence microscopy. Images (supplemental Figure 2A,B) and video (supplemental Video 1) indicate a proportion of Stx12 resides on the same organelles as SNX17. GFP-Stx11 also showed appreciable colocalization with Cherry-SNX17, while GFP-Stx17 showed minimal colocalization (supplemental Figure 2A,B).
Stx12 deficiency causes α-granule defects
We compared the levels of α-granule proteins in Stx12-deficient imMKCL cells in 2 independent ways. First, we subjected imMKCL cells to control and Stx12 siRNA treatment, generated total cell extracts, and determined the level of vWF, PF4, and P-selectin were reduced in Stx12-deficient cells as assessed by immunoblotting (Figure 2A,B). Depletion of Stx11 by siRNA did not cause a reduction in vWF, PF4, and P-selectin (supplemental Figure 2C,D), while an independent Stx12 siRNA confirmed reduced α-granule cargo (supplemental Figure 3A). Second, we generated imMKCL Stx12 KO cells using CRISPR (supplemental Figure 3B,D) and analyzed the levels of various proteins by immunofluorescence microscopy (Figure 2C). Quantification demonstrated the amount of P-selectin and PF4 was significantly reduced in the Stx12 KO cells while a control protein (TGN38) was unaffected (Figure 2D). Stx12 KO imMKCL cells also showed reduced levels of α-granule proteins by immunoblotting analysis of total cell extracts (supplemental Figure 4). Correspondingly, immunoblotting analysis of platelets generated from Stx12 KO imMKCL cells contained reduced overall levels of α-granule proteins relative to platelets generated from control imMKCL cells (supplemental Figure 5). Therefore, Stx12 but not Stx11 is required for normal steady-state levels of α-granule proteins. Additional immunoblotting analysis of Stx12 KO imMKCL cells did not show a change in overall levels of LAMP2, VMAT2, and cathepsin D, indicating δ-granules and lysosomes are not affected (supplemental Figure 6). Confirming Stx12 is needed for α-granule biogenesis, electron microscopy analysis of Stx12 KO imMKCL cells showed a significant reduction in the number of α-granules and an increase in multivesicular bodies (MVBs), which are α-granule precursors (Figure 2E,F).
Stx12 is required for normal α-granule biogenesis. (A) IB analysis of total extracts from imMKCL cells treated with either Control or Stx12 siRNA. (B) Quantification of the experiment is shown in A (n = 3). Stx12 siRNA samples were compared with Control siRNA, and statistical significance was determined via unpaired, 2-tailed Student t test. (C) Immunofluorescence microscopy analysis of wild-type (WT) or Stx12 KO imMKCL cells costained with antibodies against P-selectin, PF4, and TGN38. Bar = 5 µm. (D) Fluorescence intensity quantification of the experiment shown in C (WT, n = 50; Stx12 KO, n = 59). Stx12 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test. A.U., arbitrary units. (E) Thin-section transmission electron microscopy analysis of WT and Stx12 KO imMKCL cells subjected to high-pressure freezing. Original magnification, ×11 000 for the picture showing the entire cell and ×49 000 for the enlarged insets (bars represent 1 μm and 100 nm, respectively). (F) Quantification of α-granules, δ-granules, and MVBs visualized in the experiment shown in E (n = 12 cells per genotype). Stx12 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test. ν, nucleus; α, α-granules; δ, δ-granules; MVB, multivesicular body; mi, mitochondria.
Stx12 is required for normal α-granule biogenesis. (A) IB analysis of total extracts from imMKCL cells treated with either Control or Stx12 siRNA. (B) Quantification of the experiment is shown in A (n = 3). Stx12 siRNA samples were compared with Control siRNA, and statistical significance was determined via unpaired, 2-tailed Student t test. (C) Immunofluorescence microscopy analysis of wild-type (WT) or Stx12 KO imMKCL cells costained with antibodies against P-selectin, PF4, and TGN38. Bar = 5 µm. (D) Fluorescence intensity quantification of the experiment shown in C (WT, n = 50; Stx12 KO, n = 59). Stx12 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test. A.U., arbitrary units. (E) Thin-section transmission electron microscopy analysis of WT and Stx12 KO imMKCL cells subjected to high-pressure freezing. Original magnification, ×11 000 for the picture showing the entire cell and ×49 000 for the enlarged insets (bars represent 1 μm and 100 nm, respectively). (F) Quantification of α-granules, δ-granules, and MVBs visualized in the experiment shown in E (n = 12 cells per genotype). Stx12 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test. ν, nucleus; α, α-granules; δ, δ-granules; MVB, multivesicular body; mi, mitochondria.
Stx12 and CCDC22 compete for binding to VPS33B
We found Stx12 binds VPS16B/VPS33B via its SNARE domain (Figure 1A-E), and the C-terminal region of CCDC22 was reported to bind VPS16B/VPS33B.19 Since both the Stx12 SNARE and CCDC22 C-terminal region form coiled coils (CCs), we hypothesized they may interact with the same region of VPS16B/VPS33B and therefore compete for binding. To test this idea, Strep-Tactin beads with bound Twin Strep-VPS16B/His-VPS33B complex (VV beads) were incubated with purified GST, GST-Stx12 SNARE, GST-Stx12 Cytosolic domain, or His-CCDC22 CC domain. VV beads were able to pull down Stx12 SNARE and Cytosolic domain and CCDC22 CC as expected but not the GST control (Figure 3A, lanes 1, 2, 3, and 4). Excitingly, when coincubated with VV beads, GST-Stx12 SNARE and His-CCDC22 CC domain partially displaced each other (Figure 3A, lane 6). In agreement with the lower affinity of Stx12 Cytosolic domain for VPS16B/VPS33B relative to the Stx12 SNARE domain, the Stx12 Cytosolic domain was more effectively displaced from VV beads when coincubated with His-CCDC22 CC (Figure 3A, lane 7). For further confirmation of this result, we performed a titration experiment. VV beads were coincubated with constant amounts of His-CCDC22 CC and increasing concentrations of GST-Stx12 SNARE. His-CCDC22 CC displacement from VV beads correlated with the increase of GST-Stx12 SNARE concentration consistent with competition for binding to the VPS16B/VPS33B complex (Figure 3B-C).
Stx12 and CCDC22 compete for binding to the VPS16B/VPS33B complex. (A) Top, cartoon depicting CCDC22 coiled-coil (CC) domain. Bottom, Twin Strep pulldown. Purified Twin Strep-VPS16B/His-VPS33B complex bound to Strep-Tactin beads (VV beads) was tested for its ability to pulldown (PD) purified GST (A, lane 1), GST-Stx12 SNARE (B, lane 2), GST-Stx12 Cytosolic domain (C, lane 3), His-CCDC22 CC (lane 4) or a combination of His-CCDC22 CC together with GST (lane 5), His-CCDC22 CC together with GST-Stx12 SNARE (lane 6) and His-CCDC22 CC together with GST-Stx12 Cytosolic domain (lane 7). Bound proteins were analyzed by SDS-PAGE (Coomassie) and immunoblotting (IB) using antibodies against CCDC22 and GST. (B) VV beads were incubated with a constant amount of His-CCDC22 CC and increasing amounts of GST-Stx12 SNARE. Bound proteins were analyzed by SDS-PAGE (Coomassie). The last lane to the right represents VV beads that were incubated with buffer only (no His-CCDC22 CC or GST-Stx12 SNARE). (C) His-CCDC22 CC band intensity quantification of the experiment shown in B (n = 2).
Stx12 and CCDC22 compete for binding to the VPS16B/VPS33B complex. (A) Top, cartoon depicting CCDC22 coiled-coil (CC) domain. Bottom, Twin Strep pulldown. Purified Twin Strep-VPS16B/His-VPS33B complex bound to Strep-Tactin beads (VV beads) was tested for its ability to pulldown (PD) purified GST (A, lane 1), GST-Stx12 SNARE (B, lane 2), GST-Stx12 Cytosolic domain (C, lane 3), His-CCDC22 CC (lane 4) or a combination of His-CCDC22 CC together with GST (lane 5), His-CCDC22 CC together with GST-Stx12 SNARE (lane 6) and His-CCDC22 CC together with GST-Stx12 Cytosolic domain (lane 7). Bound proteins were analyzed by SDS-PAGE (Coomassie) and immunoblotting (IB) using antibodies against CCDC22 and GST. (B) VV beads were incubated with a constant amount of His-CCDC22 CC and increasing amounts of GST-Stx12 SNARE. Bound proteins were analyzed by SDS-PAGE (Coomassie). The last lane to the right represents VV beads that were incubated with buffer only (no His-CCDC22 CC or GST-Stx12 SNARE). (C) His-CCDC22 CC band intensity quantification of the experiment shown in B (n = 2).
To investigate whether Stx12 and CCDC22 bind to the same region of VPS16B/VPS33B, we created internal deletion mutants of VPS33B. Deletions were engineered based on the available data for yeast Vps33.43 We generated a 3-dimensional structure of VPS33B using homology modeling and generated 2 deletions mapping on the helical hairpin predicted to bind SNARE domains (Figure 4A). The smaller deletion comprises amino acids 314 to 336 (pink), and the larger deletion consists of amino acids 308 to 345, which includes the smaller one (yellow + pink). Gel filtration analysis suggested VPS33B deletions did not affect binding to VPS16B or altered overall folding (Figure 4B). Both deletions drastically diminished the binding of GST-Stx12 SNARE and His-CCDC22 CC to the VPS16B/VPS33B complex (Figure 4C), revealing Stx12 and CCDC22 compete for bind to the same region of VPS33B.
Stx12 and CCDC22 bind to the same region of VPS33B, which is essential for α-granule biogenesis. (A) Model of the human VPS33B 3-dimensional structure based on the human VPS33A crystal structure (PDB code: 4BX9).50 Deletions in the helical hairpin region of VPS33B are indicated in pink (Δ314-336) and yellow (Δ308-345). (B) Gel filtration analysis of purified WT or mutants Twin Strep-VPS16B/His-VPS33B complexes. (C) VV beads containing WT or the different VPS33B deletions were tested for their ability to pulldown GST-Stx12 SNARE or His-CCDC22 CC. Bound proteins were analyzed by IB using antibodies against GST and CCDC22 as well as by SDS-PAGE (Coomassie). (D) IB analysis of total cell extracts of VPS33B KO cells expressing GFP, GFP-VPS33B WT, or GFP-VPS33B Δ314-336. (E) Quantification of the vWF and VPS16B IB band intensities from the experiment shown in panel D. The ratio of the band intensity of either VPS33B Δ314-336 or VPS33B WT samples over the VPS33B WT sample for both vWF and VPS16B proteins were compared, and statistical significance was determined via unpaired, 2-tailed Student t test (n = 2). ns, not significantly different from WT.
Stx12 and CCDC22 bind to the same region of VPS33B, which is essential for α-granule biogenesis. (A) Model of the human VPS33B 3-dimensional structure based on the human VPS33A crystal structure (PDB code: 4BX9).50 Deletions in the helical hairpin region of VPS33B are indicated in pink (Δ314-336) and yellow (Δ308-345). (B) Gel filtration analysis of purified WT or mutants Twin Strep-VPS16B/His-VPS33B complexes. (C) VV beads containing WT or the different VPS33B deletions were tested for their ability to pulldown GST-Stx12 SNARE or His-CCDC22 CC. Bound proteins were analyzed by IB using antibodies against GST and CCDC22 as well as by SDS-PAGE (Coomassie). (D) IB analysis of total cell extracts of VPS33B KO cells expressing GFP, GFP-VPS33B WT, or GFP-VPS33B Δ314-336. (E) Quantification of the vWF and VPS16B IB band intensities from the experiment shown in panel D. The ratio of the band intensity of either VPS33B Δ314-336 or VPS33B WT samples over the VPS33B WT sample for both vWF and VPS16B proteins were compared, and statistical significance was determined via unpaired, 2-tailed Student t test (n = 2). ns, not significantly different from WT.
To better understand the functional importance of VPS33B binding to Stx12 and CCDC22 in live cells, we designed a rescue experiment with VPS33B KO cells using GFP-VPS33B wild-type (WT) or the Δ314-336 mutant (Figure 4D-E). Both versions of GFP-VPS33B rescued the VPS16B defect indicating proper assembly of the VPS16B/GFP-VPS33B complex. However, unlike WT GFP-VPS33B, GFP-VPS33B Δ314-336 was not able to recover the α-granule protein vWF deficiency, indicating this VPS33B region is crucial for its role in α-granule biogenesis. Thus, the data suggests binding to Stx12 and/or CCDC22 is key for the function of VPS16B/VPS33B in α-granule biogenesis.
CCDC22 deficiency results in lower levels of α-granule proteins
Binding of CCDC22 to VPS33B and competition with Stx12 suggests CCDC22 is involved in α-granule biogenesis. To test this directly, we subjected imMKCL cells to control and CCDC22 siRNA knockdown, generated total cell extracts, and analyzed them by immunoblotting. As expected, we found that other members of the CCC complex were destabilized in CCDC22-deficient cells (supplemental Figure 7). Importantly, the levels of α-granule proteins vWF and P-selectin were diminished in these cells showing that CCDC22 and, by extension, the CCC complex functions in α-granule biogenesis.
COMMD3 defines a major form of the CCC complex
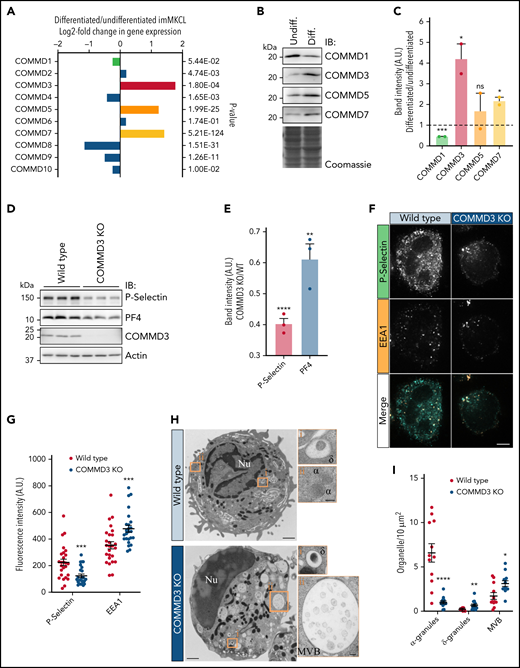
When imMKCL cells are switched from standard proliferation culture conditions to differentiation medium, they dramatically increase the expression of genes involved in α-granule and platelet production and function.15,29,41 Four independent replicates of total RNA samples from undifferentiated and differentiated imMKCL were analyzed by RNAseq. Consistently, gene ontology (GO) enrichment analysis revealed the most overrepresented GO terms include endosomal components and platelet function (supplemental Figure 8; supplemental Files 1 and 2). Excitingly, we found that the expression of only 3 COMMD genes, COMMD3, COMMD5, and COMMD7, was upregulated (Figure 5A). When protein levels were corroborated by immunoblotting, all 3 proteins appeared more highly expressed in differentiated imMKCL cells, but only COMMD3 and COMMD7 reached statistical significance while COMMD1 showed lower expression (Figure 5B-C). We then investigated the effect that siRNA knockdown of the upregulated COMMD proteins had on the stability of other components of the CCC complex. Depletion of COMMD3 (and to a lesser extent COMMD1) was most effective at diminishing the levels of CCDC22, CCDC93, VPS35L, and other COMMD proteins, although we note that depletion of COMMD5 and COMMD7 was statistically significant but less effective than depletion of COMMD3 (supplemental Figure 9). The data suggests COMMD3 is a central component of CCC in cells actively producing α-granules.
COMMD3 defines a major form of the CCC complex in MK cells and is required for normal α-granule biogenesis. (A) RNAseq analysis for the 10 COMMD genes indicates COMMD3, COMMD5, and COMMD7 are more highly expressed in differentiated than in undifferentiated imMKCL cells. (B) IB analysis of total extracts of undifferentiated and differentiated imMKCL cells. (C) Quantification of the results obtained in (B). Differentiated samples were compared with undifferentiated samples, and statistical significance was determined via unpaired, 2-tailed Student t test (n = 2). (D) Total extracts of WT or COMMD3 KO imMKCL cells were prepared and subjected to IB analysis with the indicated antibodies. Three independent experiments per cell type are shown. (E) Quantification of the results obtained in (D) expressed as the ratio of the normalized band intensities of P-selectin or PF4 in COMMD3 KO cells relative to WT imMKCL cells. COMMD3 KO samples were compared with WT samples, and statistical significance was determined via unpaired, 2-tailed Student t test (n = 3). (F) Immunofluorescence microscopy analysis of WT or COMMD3 KO cells costained with P-selectin and EEA1 antibodies. Bar = 5 µm. (G) Fluorescence intensity quantification of the cells shown in (F). COMMD3 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test (WT, n = 26; COMMD3 KO, n = 25). A.U., arbitrary units. (H) Thin-section transmission electron microscopy analysis of WT and COMMD3 KO imMKCL cells subjected to high-pressure freezing. Original magnification, ×11 000 for the picture showing the entire cell and ×49 000 for the enlarged insets (bars represent 1 μm and 100 nm, respectively). (I) Quantification of α-granules, δ-granules, and MVBs visualized in the experiment shown in (H). COMMD3 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test (WT n = 12; COMMD3 KO n = 13 cells). The WT data set is the same as the one shown in Figure 2F. ν, nucleus; α, α-granules; δ, δ-granules; MVB, multivesicular body.
COMMD3 defines a major form of the CCC complex in MK cells and is required for normal α-granule biogenesis. (A) RNAseq analysis for the 10 COMMD genes indicates COMMD3, COMMD5, and COMMD7 are more highly expressed in differentiated than in undifferentiated imMKCL cells. (B) IB analysis of total extracts of undifferentiated and differentiated imMKCL cells. (C) Quantification of the results obtained in (B). Differentiated samples were compared with undifferentiated samples, and statistical significance was determined via unpaired, 2-tailed Student t test (n = 2). (D) Total extracts of WT or COMMD3 KO imMKCL cells were prepared and subjected to IB analysis with the indicated antibodies. Three independent experiments per cell type are shown. (E) Quantification of the results obtained in (D) expressed as the ratio of the normalized band intensities of P-selectin or PF4 in COMMD3 KO cells relative to WT imMKCL cells. COMMD3 KO samples were compared with WT samples, and statistical significance was determined via unpaired, 2-tailed Student t test (n = 3). (F) Immunofluorescence microscopy analysis of WT or COMMD3 KO cells costained with P-selectin and EEA1 antibodies. Bar = 5 µm. (G) Fluorescence intensity quantification of the cells shown in (F). COMMD3 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test (WT, n = 26; COMMD3 KO, n = 25). A.U., arbitrary units. (H) Thin-section transmission electron microscopy analysis of WT and COMMD3 KO imMKCL cells subjected to high-pressure freezing. Original magnification, ×11 000 for the picture showing the entire cell and ×49 000 for the enlarged insets (bars represent 1 μm and 100 nm, respectively). (I) Quantification of α-granules, δ-granules, and MVBs visualized in the experiment shown in (H). COMMD3 KO samples were compared with WT samples, and statistical significance was determined via unpaired, Mann-Whitney U test (WT n = 12; COMMD3 KO n = 13 cells). The WT data set is the same as the one shown in Figure 2F. ν, nucleus; α, α-granules; δ, δ-granules; MVB, multivesicular body.
COMMD3 deficiency results in α-granule defects
To test for a functional role of COMMD3 in α-granule biogenesis, we generated COMMD3 KO cells using CRISPR (supplemental Figure 3C,D). Immunoblotting analysis of total extracts from 3 independent replicates indicated COMMD3 KO cells contained reduced levels of P-selectin and PF4 (Figure 5D-E) as well as other CCC components (supplemental Figure 10). Furthermore, quantification of immunofluorescence microscopy images also showed that COMMD3 KO cells contain less P-selectin than WT cells (Figure 5F-G). By contrast, the fluorescence intensity of endosomal marker EEA1 was slightly increased in the COMMD3 KO imMKCL cells, as previously shown in Hela cells.16 Additionally, immunoblotting analysis of platelets generated from COMMD3 KO imMKCL cells contained reduced overall levels of α-granule proteins relative to platelets generated from control imMKCL cells (supplemental Figure 5). These data support the concept that COMMD3 and the CCC complex function in α-granule biogenesis. Confirming COMMD3 is needed for α-granule biogenesis, electron microscopy analysis of COMMD3 KO imMKCL cells showed a significant reduction in the number of α-granules and an increase in the numbers of MVBs (which were also dramatically larger) and δ-granules (Figure 5H-I). Further immunoblotting analysis of COMMD3 KO imMKCL cells showed increased overall levels of VMAT2 and LAM2, consistent with the increased numbers of δ-granules (supplemental Figure 6).
Interestingly, the levels of the CCC complex proteins CCDC22, COMMD3, and COMMD1 are increased in VPS33B KO imMKCL cells compared with WT cells (supplemental Figure 11), suggesting a compensatory mechanism and functional connection between CCC and the VPS16B/VPS33B complex.
COMMD3 deficiency results in degradation of α-granule proteins
We previously showed that deficiency of VPS33B causes α-granule proteins to follow a degradative pathway.15 To investigate whether COMMD3 deficiency causes a similar defect, we tested if the vacuolar H+ ATPase inhibitor BafA1 would rescue the steady-state levels of α-granule proteins. We treated WT and COMMD3 KO cells with BafA1 or vehicle (DMSO) and fixed and stained the cells with anti-PF4 and anti-P-selectin antibodies. WT cells showed no significant difference in fluorescence intensity between BafA1 and control cells for any of the markers. However, BafA1 treatment rescued the fluorescence intensity of PF4 and P-selectin in COMMD3 KO cells (Figure 6A-B). An analogous result was observed by immunoblotting analysis of total cell extracts. Overall levels of PF4 and P-selectin were unperturbed by BafA1 treatment in WT cells and Stx12 KO cells, but the same treatment increased the α-granule protein levels in COMMD3 KO cells (supplemental Figure 12). These results indicate that both soluble and transmembrane α-granule proteins require COMMD3 to avoid a degradative pathway.
COMMD3 deficiency results in α-granule protein following a degradative pathway, and the COMMD3 protein localizes to Stx12-positive endosomal subdomains. (A) Immunofluorescence microscopy analysis of WT and COMMD3 KO imMKCL cells treated with either vehicle (DMSO) or BafA1 and costained with P-selectin and PF4 antibodies. Bar = 5 µm. (B) Fluorescence intensity quantification of the cells shown in (A). Statistical significance was determined via unpaired, Mann-Whitney U test (WT DMSO, n = 27; WT BafA1, n = 35; COMMD3 KO DMSO, n = 38; COMMD3 KO BafA1, n = 57). A.U., arbitrary units. (C) Spinning disk confocal microscopy analysis of a COMMD3 KO imMKCL cell expressing GFP-COMMD3 and BFP-Stx12. Bar = 5 µm. (D) Quantification of BFP-Stx12 and GFP-COMMD3 colocalization of the cells shown in C (n = 25). (E) Left, superresolution scanning confocal Airyscan image of a COMMD3 KO imMKCL cell expressing GFP-COMMD3 and Cherry-Stx12 (bar = 5 µm). Right, multichannel view magnification of the region indicated by the dashed line rectangle (bar = 1 µm). The filled triangles indicate endosomal subdomains where GFP-COMMD3 and Cherry-Stx12 colocalize, while open triangles point toward Cherry-Stx12 endosomal tubules, which are COMMD3-negative but are continuous with GFP-COMMD3-positive subdomains. Supplemental Figure 16 shows additional examples of superresolution scanning confocal Airyscan images of COMMD3 KO imMKCL cells expressing GFP-COMMD3 and Cherry-Stx12.
COMMD3 deficiency results in α-granule protein following a degradative pathway, and the COMMD3 protein localizes to Stx12-positive endosomal subdomains. (A) Immunofluorescence microscopy analysis of WT and COMMD3 KO imMKCL cells treated with either vehicle (DMSO) or BafA1 and costained with P-selectin and PF4 antibodies. Bar = 5 µm. (B) Fluorescence intensity quantification of the cells shown in (A). Statistical significance was determined via unpaired, Mann-Whitney U test (WT DMSO, n = 27; WT BafA1, n = 35; COMMD3 KO DMSO, n = 38; COMMD3 KO BafA1, n = 57). A.U., arbitrary units. (C) Spinning disk confocal microscopy analysis of a COMMD3 KO imMKCL cell expressing GFP-COMMD3 and BFP-Stx12. Bar = 5 µm. (D) Quantification of BFP-Stx12 and GFP-COMMD3 colocalization of the cells shown in C (n = 25). (E) Left, superresolution scanning confocal Airyscan image of a COMMD3 KO imMKCL cell expressing GFP-COMMD3 and Cherry-Stx12 (bar = 5 µm). Right, multichannel view magnification of the region indicated by the dashed line rectangle (bar = 1 µm). The filled triangles indicate endosomal subdomains where GFP-COMMD3 and Cherry-Stx12 colocalize, while open triangles point toward Cherry-Stx12 endosomal tubules, which are COMMD3-negative but are continuous with GFP-COMMD3-positive subdomains. Supplemental Figure 16 shows additional examples of superresolution scanning confocal Airyscan images of COMMD3 KO imMKCL cells expressing GFP-COMMD3 and Cherry-Stx12.
COMMD3 localizes to a subdomain of Stx12 endosomes
We coexpressed GFP-COMMD3 and BFP2-Stx12 in COMMD3 KO imMKCL cells. Spinning disk confocal microscopy showed a proportion of both proteins localizes to the same organelles (Figure 6C) and move together (supplemental Video 2). However, BFP2-Stx12 and GFP-COMMD3 display different localization patterns, with GFP-COMMD3 labeling a small region of the BFP2-Stx12-labeled structures. This is likely reflected in the lower-than-expected colocalization quantified as Manders’ overlap coefficient of 0.37 ± 0.01 (Figure 6D). Intrigued by this result, we analyzed these cells using a superresolution scanning confocal microscope. Airyscan images show Cherry-STX12 is present in vacuolar domains and in thin tubules emanating from vacuolar domains (Figure 6E). Excitingly, GFP-COMMD3 specifically decorates the region where BFP2-Stx12 vacuolar domains connect with tubules (Figure 6 inset, filled triangles), and even though most Stx12-containing vacuolar domains are connected to several tubules, GFP-COMMD3 only labels a subset of tubules. This result indicates a high degree of specialization of endosomal subdomains and suggests these specific tubules are used by the α-granule proteins during α-granule biogenesis.
P-selectin is an SNX17 cargo and traffics through the plasma membrane in a COMMD3-dependent manner
P-selectin contains a sorting signal in its cytosolic domain that is bound by the endosomal adaptor protein SNX17.26,27,44 Here, we investigated whether P-selectin relies on SNX17 for its traffic to α-granules. We coexpressed P-selectin-GFP and SNX17 with a Cherry tag fused to either its N or C-terminus. While Cherry-SNX17 localizes and functions normally, SNX17-Cherry is not functional, although it maintains normal localization and the ability to bind cargo, thus acting as a dominant-negative (DN) form.24 Confocal microscopy images show that although P-selectin-GFP and Cherry-SNX17 partially colocalize in sorting endosomes, a significant cohort of P-selectin-GFP is present in different structures, presumably α-granules (Figure 7A-B). However, when P-selectin-GFP is coexpressed with SNX17-Cherry, the DN form, they show stronger colocalization, indicating P-selectin-GFP is retained in endosomes (Figure 7A-B). This result indicates P-selectin requires a functional SNX17 for proper trafficking to α-granules.
P-selectin is an SNX17 cargo and traffics through the plasma membrane in a COMMD3-dependent manner. (A) Spinning disk confocal fluorescence microscopy images of imMKCL cells expressing P-selectin WT or F826G-GFP and Cherry-SNX17 or SNX17-Cherry (DN). Bar = 5 µm. (B) Colocalization analysis of the cells shown in A. Statistical significance was determined via unpaired, 2-tailed Student t test (P-selectin WT+Cherry-SNX17, n = 25; P-selectin WT+SNX17-Cherry [DN], n = 29; P-selectin F826G+SNX17-Cherry [DN], n = 11). DN, dominant negative. (C) Confocal fluorescence microscopy images of WT and COMMD3 KO imMKCL cells that were incubated with primary antibodies against P-selectin, CD71, and SNX17 and subsequently fixed, permeabilized, and stained with species-specific secondary antibodies conjugated with Alexa Fluor 488, 546, and 647, respectively. To help visualization, the edge of some cells has been drawn with a dotted line. Bar = 5 µm. (D) Quantification of fluorescence intensity of internalized anti-P-selectin, CD71, and SNX17 antibodies in WT and COMMD3 KO imMKCL cells. Statistical significance was determined via unpaired, Mann-Whitney U test (WT, n = 99; COMMD3 KO, n = 76). While detection of SNX17 is negligible in both samples, it was slightly higher in COMMD3 KO cells. A.U., arbitrary units.
P-selectin is an SNX17 cargo and traffics through the plasma membrane in a COMMD3-dependent manner. (A) Spinning disk confocal fluorescence microscopy images of imMKCL cells expressing P-selectin WT or F826G-GFP and Cherry-SNX17 or SNX17-Cherry (DN). Bar = 5 µm. (B) Colocalization analysis of the cells shown in A. Statistical significance was determined via unpaired, 2-tailed Student t test (P-selectin WT+Cherry-SNX17, n = 25; P-selectin WT+SNX17-Cherry [DN], n = 29; P-selectin F826G+SNX17-Cherry [DN], n = 11). DN, dominant negative. (C) Confocal fluorescence microscopy images of WT and COMMD3 KO imMKCL cells that were incubated with primary antibodies against P-selectin, CD71, and SNX17 and subsequently fixed, permeabilized, and stained with species-specific secondary antibodies conjugated with Alexa Fluor 488, 546, and 647, respectively. To help visualization, the edge of some cells has been drawn with a dotted line. Bar = 5 µm. (D) Quantification of fluorescence intensity of internalized anti-P-selectin, CD71, and SNX17 antibodies in WT and COMMD3 KO imMKCL cells. Statistical significance was determined via unpaired, Mann-Whitney U test (WT, n = 99; COMMD3 KO, n = 76). While detection of SNX17 is negligible in both samples, it was slightly higher in COMMD3 KO cells. A.U., arbitrary units.
We then introduced a point mutation (F826G) in the P-selectin sorting signal bound by SNX17.25 P-selectin F826G is no longer retained in SNX17-Cherry positive endosomes, and it is readily detectable at the plasma membrane (Figure 7A-B). This result further suggests P-selectin dependence on binding to SNX17 for proper transport to α-granules and that P-selectin may normally traffic through the cell surface.
In nonspecialized cells, CCC and other protein complexes work together facilitating the recycling of SNX17 cargo from endosomes to the cell surface.24 Given the results with P-selectin and SNX17 described above, we wondered if this pathway is involved in α-granule biogenesis. To test this hypothesis, we performed 2 experiments. First, we investigated whether endogenous P-selectin traffics through the plasma membrane in WT megakaryocytes. We isolated primary MKs from mouse bone marrow (BM) and incubated the live cells with a FITC-conjugated anti-P-selectin antibody that recognizes the extracellular/organelle luminal portion of P-selectin and, as a control, an irrelevant AF647-conjugated antibody. Fluorescence microscopy analysis showed the anti-P-selectin antibody (but not the control antibody) in internal puncta (supplemental Figure 13). This indicates P-selectin normally traffics through the megakaryocyte plasma membrane but is rapidly endocytosed. Second, we tested whether P-selectin trafficking through the plasma requires the COMMD3/CCC complex. We incubated live WT and COMMD3 KO imMKCL cells with an antibody that recognizes the extracellular/organelle luminal portion of P-selectin, washed, fixed/permeabilized, and probed the cells with a fluorescent secondary antibody. Fluorescence microscopy analysis showed a dramatic reduction of internalized anti-P-selectin antibodies in COMMD3 KO cells (Figure 7C-D). As controls, parallel analysis with anti-CD71 antibody showed no defect in COMMD3 KO cells, and anti-SNX17 antibody was not internalized in control or COMMD3 KO cells. Therefore, P-selectin is an SNX17 cargo and requires COMMD3 for normal trafficking through the megakaryocyte plasma membrane.
Discussion
Our understanding of how platelet α-granules are made is in its infancy, with limited knowledge of the cellular mechanisms and molecular machinery involved.1,4,5 On the one hand, there is a significant paucity of proteins that mediate α-granule biogenesis. On the other hand, while VPS16B and VPS33B are 2 well-established components of the machinery that form a stable protein complex, how they accomplish their work is poorly understood. Here we uncovered Stx12 and COMMD3/CCC as new players of the α-granule biogenesis machinery that interact physically and functionally with the VPS16B/VPS33B complex to mediate the formation of α-granules. However, the phenotypes of Stx12 and COMMD3/CCC deficiency are less penetrant than that of VPS16B/VPS33B deficiency, suggesting a subordinate role in α-granule biogenesis.
In previous work, we found the VPS16B/VPS33B complex primarily localizes at a subset of endosomes where α-granule proteins are sorted in a pathway toward α-granules and away from a degradative pathway.15 Given that VPS33B is classified as an SM protein, it has been hypothesized that it regulates a SNARE-mediated vesicle fusion step.14 Here, we found that the VPS16B/VPS33B complex preferentially binds the Qa-SNARE Stx12, which localizes, at least in part, to endosomal compartments. Given that Qa-SNAREs typically localize at the target compartment,38 the data suggests VPS16B/VPS33B regulates the Stx12-mediated fusion of vesicles arriving at endosomes. VPS16B/VPS33B binds the Stx12 SNARE domain more strongly than the entire cytosolic region, indicating Stx12 adopts a closed conformation. This suggests a model akin to other syntaxins45 in which a priming factor may be required for Stx12 to achieve a functional/open state, further supporting Stx12 function in α-granule biogenesis and lack of Stx12 results in α-granule deficiency in imMKCL cells and platelets. However, this phenotype is less severe than the one observed with VPS33B KO imMKCL cells,15 perhaps due to SNARE redundancy.
The CCC complex component CCDC22 was recently found to bind VPS16B/VPS33B via its CC domain,19 raising the possibility that in megakaryocytes, CCC may be part of the α-granule biogenesis machinery. We reproduced that physical interaction here. More importantly, we discovered that the CCDC22 CC domain competes with the SNARE domain of Stx12 for binding to a region of VPS33B that is important for VPS33B function in α-granule biogenesis. We also found COMMD3 is upregulated and defines a major form of the CCC complex in cells actively producing α-granules and that COMMD3 and CCDC22 deficiency results in α-granule defects. Together, the data establishes the COMMD3/CCC complex as a new factor functioning in α-granule biogenesis.
In nonspecialized cells, the CCC complex, together with the cargo adaptor SNX17 and the Retriever and WASH complexes, mediate the formation of tubular carriers that exit endosomes and retrieve cargo away from a degradative pathway.16-18 Our data indicates CCC likely performs an analogous function during α-granule biogenesis in megakaryocytes. The fact that CCDC22 and Stx12 compete for binding to VPS33B may represent a physical connection between the entry and the exit of α-granule cargo at endosomes. The data fit a hand-off mechanism in which α-granule protein cargo arrives at endosomes in vesicles that fuse at Stx12-VPS16B/VPS33B sites, and after fusion occurs, it is transferred to the COMMD3/CCC retrieval machinery, thus ensuring it escapes a degradative fate. Supporting this model, we found COMMD3 marks a subset of tubules emanating from Stx12-positive endosome vacuolar domains. Thus, COMMD3/CCC likely defines specific endosome exit sites that function in α-granule biogenesis.
P-selectin binds the endosomal adaptor SNX17,44 but the importance of that connection has not been specifically investigated in megakaryocytes.46,47 Here, we show that P-selectin is retained in endosomes in the presence of a DN SNX17 form, and it accumulates at the cell surface when its SNX17 sorting signal is mutated. This implies that P-selectin requires SNX17 to exit endosomes and suggests that it traffics through the plasma membrane before arriving at the α-granule. We established endogenous P-selectin indeed traffics through the cell surface of both primary mouse megakaryocytes and imMKCL cells by incubating live cells with antibodies to the extracellular region of P-selectin. We found the anti-P-selectin antibody in internal structures but did not detect it on the cell surface, consistent with rapid endocytosis and minimal surface expression at steady state. Although the passage of P-selectin through the cell surface could be alternatively explained by α-granule fusion with the megakaryocyte plasma membrane, this is unlikely because it would cause the release of soluble content into the BM milieu. Additionally, a recent paper reported PF4 is not normally secreted from isolated mouse primary megakaryocytes in culture.48 Importantly, we found that P-selectin trafficking through the plasma membrane depends on COMMD3, indicative of an endosome-plasma membrane recycling pathway that P-selectin probably goes through before reaching α-granules. Interestingly, retromer, an endosomal complex that is structurally and functionally related to the retriever complex,17 regulates the trafficking of melanogenic enzymes to the melanosome in melanocytes.49 Like α-granules, melanosomes are lysosome-related organelles,6 suggesting endosomal retrieval complexes are repurposed for the formation of lysosome-related organelles in specialized cells.
In summary, we have revealed new factors (Stx12, COMMD3/CCC, SNX17) that work in concert with VPS16B/VPS33B during the biogenesis of α-granules, identifying them as candidates for platelet-type bleeding disorders of unknown molecular cause. Our results reinforce the central role played by endosomes in α-granule biogenesis and suggest a mechanism by which VPS16B/VPS33B coordinates the α-granule cargo entry to and exit from endosomes. More broadly, the results imply megakaryocytes have adapted the ubiquitous endosomal trafficking and retrieval machinery to synthesize α-granules.
Acknowledgments
The authors thank Koji Eto (Kyoto University) for the imMKCLs; Brett Collins (University of Queensland) for an inspiring discussion; and Robert Cohen, Tinting Yao, and Carolina Dos Santos Passos (Colorado State University) for their help with the Airyscan imaging. This work was supported by National Heart, Lung, and Blood Institute grant R01HL151988 and National Institute of General Medical Sciences grant R01GM125619 (S.M.D.P.). The confocal microscope used in this work is supported in part by the CSU Microscope Imaging Network core infrastructure grant.
Authorship
Contribution: A.L.A. and S.M.D.P. designed the study, performed the experiments, analyzed the data, and wrote the manuscript; and H.P.F. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Santiago M. Di Pietro, Department of Biochemistry and Molecular Biology, Colorado State University, Fort Collins, CO 80523-1870; e-mail: santiago.dipietro@colostate.edu.
Requests for data sharing may be submitted to Santiago M. Di Pietro (santiago.dipietro@colostate.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.






![P-selectin is an SNX17 cargo and traffics through the plasma membrane in a COMMD3-dependent manner. (A) Spinning disk confocal fluorescence microscopy images of imMKCL cells expressing P-selectin WT or F826G-GFP and Cherry-SNX17 or SNX17-Cherry (DN). Bar = 5 µm. (B) Colocalization analysis of the cells shown in A. Statistical significance was determined via unpaired, 2-tailed Student t test (P-selectin WT+Cherry-SNX17, n = 25; P-selectin WT+SNX17-Cherry [DN], n = 29; P-selectin F826G+SNX17-Cherry [DN], n = 11). DN, dominant negative. (C) Confocal fluorescence microscopy images of WT and COMMD3 KO imMKCL cells that were incubated with primary antibodies against P-selectin, CD71, and SNX17 and subsequently fixed, permeabilized, and stained with species-specific secondary antibodies conjugated with Alexa Fluor 488, 546, and 647, respectively. To help visualization, the edge of some cells has been drawn with a dotted line. Bar = 5 µm. (D) Quantification of fluorescence intensity of internalized anti-P-selectin, CD71, and SNX17 antibodies in WT and COMMD3 KO imMKCL cells. Statistical significance was determined via unpaired, Mann-Whitney U test (WT, n = 99; COMMD3 KO, n = 76). While detection of SNX17 is negligible in both samples, it was slightly higher in COMMD3 KO cells. A.U., arbitrary units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/6/10.1182_blood.2021012056/3/m_bloodbld2021012056f7.png?Expires=1767795494&Signature=pfx2aAzttcRuvWU9ncVOh4C5DRBEQ7BpJ~tzRHQYz4Ok-fSJNXK2-q3JlWCpcsMNZfHuEeZCmmQQExn9zIdgA77LbGttctz1agHViQ-KoRM~g4MqILpC5e1rBXsOXHhBh43a8IxoAt6oIOT2qc07Pr5k3Em-xTbrBOXujFsdNNOqDkXL2KMHdxKs8xZ8IAMDIUrrmpPMNuva2gSSPlnhOb6OvOs7BC4LOCThDKBHHyazVmYD5sTaXUUrYSqgYes9mAB1f9cS5QjoskG1aa6Sl7Zqjajm-G8HuZeCYgtwj9iTKPLy-aNZA3YkeQQRpoDjpLMrvNuGh1etAtsGjUPfDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal