Abstract
Diffuse large B-cell lymphoma (DLBCL), the most common lymphoma subtype, is localized in 25% to 30% of patients. Prognosis in patients with limited-stage DLBCL (LS-DLBCL) is excellent with 10-year overall survival of at least 70% to 80%. Improved insights into the disease biology, the availability of positron-emission tomography (PET) scans, and recent dedicated clinical trials within this unique population have led to evolving treatment paradigms. However, no standard definition of LS-DLBCL exists, and although generally defined as Ann Arbor stages I to II disease with largest mass size <10 cm in diameter, variations across studies cause challenges in interpretation. Similar to advanced-stage disease, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) immunochemotherapy forms the basis of treatment, with combined modality therapy including 3 cycles of systemic treatment and involved-site radiation therapy being a predominant historical standard. Yet the well-described continuous risk of relapse beyond 5 years and established late complications of radiotherapy have challenged previous strategies. More rigorous baseline staging and response assessment with PET may improve decision making. Recent clinical studies have focused on minimizing toxicities while maximizing disease outcomes using strategies such as abbreviated immunochemotherapy alone and PET-adapted radiotherapy delivery. This comprehensive review provides an update of recent literature with recommendations for integration into clinical practice for LS-DLBCL patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common lymphoma, is localized at presentation in 25% to 30% of patients.1-3 No standardized definition of localized disease exists, nor does a global standard approach to prognostication and management. Conventionally, localized, early or limited-stage DLBCL (LS-DLBCL) has been defined as Ann Arbor stages I-II nonbulky disease (commonly <10 cm maximum tumor dimension). The advent of routine 18F-fluorodeoxyglucose positron-emission tomography (PET) staging has further honed identification of LS-DLBCL, and molecular testing advances have contributed to better understanding of the biology. Prognosis is generally excellent; however, application of risk stratification used in advanced-stage disease is challenging due to the absence of common adverse features. Despite few randomized studies, numerous treatment strategies aiming to minimize toxicity while maintaining excellent outcomes have emerged and proven successful. Variability in patient presentation can complicate treatment selection, although the role of radiotherapy (RT) in the rituximab era is being increasingly questioned. This overview addresses current evidence and its impact on modern routine care of LS-DLBCL. Specific circumstances including testicular, primary mediastinal, and central nervous system (CNS) lymphomas are excluded from the discussion due to their individualized approach.
Diagnosis and staging
Standard diagnosis for DLBCL applies with excisional biopsy and expert hematopathologist review essential to ensure adequate tissue for diagnostic evaluation and appropriate molecular classification. Core biopsy should only be considered when excisional biopsy is not readily feasible.4,5 Diagnosis is according to the World Health Organization (WHO) 2016 criteria with LS-DLBCL presenting identically to advanced-stage disease with a diffuse proliferation of mature large B cells.6
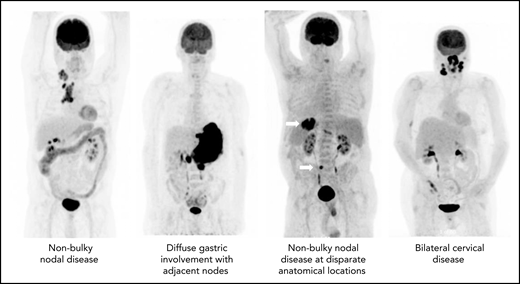
The precise definition of LS-DLBCL is not standardized. Although it is clear that localized disease is restricted to Ann Arbor stages I to II, variation among studies exists in definition of bulk (maximum dimension ranging between 5 and 10 cm), inclusion of B symptoms, and staging methodology. Although not always explicitly stated, LS-DLBCL generally implies disease that can be readily encompassed within a reasonable RT field, as historically RT was considered a mainstay of therapy (Figure 1).7-12
The diagnostic and staging work up for LS-DLBCL should follow standard DLBCL recommendations including baseline clinical and organ function assessments plus Ann Arbor staging (Table 1). Accurate identification of LS-DLBCL has significant treatment implications, particularly in patients being considered for abbreviated therapy. PET plus computed tomography (PET-CT) is recommended for all DLBCL patients due to its superiority in identifying disease over standard contrast-enhanced CT, with PET up-staging 5% to 15% of DLBCL.13,14 The value of bone marrow biopsy (BMB) has been scrutinized in the PET-era and is listed as optional in the Lugano staging recommendations.13 PET-CT detects bone marrow involvement with high sensitivity and specificity (86% and 100%, respectively) but can miss the rare presence of indolent or low-volume disease,15 with 2% to 6% of patients with PET-determined LS-DLBCL having demonstrable marrow involvement detected exclusively by BMB, thus up-staging to stage IV disease.16-18 Thus, BMB is preferred for more accurate staging, especially if abbreviated therapy is being considered.
Recommended diagnostic evaluation
| Baseline information . | Tests required . |
|---|---|
| Histological diagnosis via excisional biopsy preferred | • According to WHO criteria, including COO phenotype (gene expression profiling or IHC) and FISH for MYC, BCL2, and BCL6 |
| Clinical assessment | • Age • Performance status • B symptoms • Comorbidities |
| Staging Including documentation of: Ann Arbor stage Extranodal sites Presence of bulk | • PET-CT • Other radiological tests as indicated • Bone marrow biopsy (optional but preferred) |
| Organ function | • Laboratory tests: ○ Lactate dehydrogenase (LDH) ○ Complete blood count ○ Urea, creatinine and electrolytes ○ Liver function tests ○ Hepatitis B and C serology ○ HIV serology • Left ventricular ejection fraction: transthoracic echocardiogram or gated cardiac blood pool scan |
| Baseline information . | Tests required . |
|---|---|
| Histological diagnosis via excisional biopsy preferred | • According to WHO criteria, including COO phenotype (gene expression profiling or IHC) and FISH for MYC, BCL2, and BCL6 |
| Clinical assessment | • Age • Performance status • B symptoms • Comorbidities |
| Staging Including documentation of: Ann Arbor stage Extranodal sites Presence of bulk | • PET-CT • Other radiological tests as indicated • Bone marrow biopsy (optional but preferred) |
| Organ function | • Laboratory tests: ○ Lactate dehydrogenase (LDH) ○ Complete blood count ○ Urea, creatinine and electrolytes ○ Liver function tests ○ Hepatitis B and C serology ○ HIV serology • Left ventricular ejection fraction: transthoracic echocardiogram or gated cardiac blood pool scan |
FISH, fluorescence in situ hybridization; PET-CT, positron-emission tomography with computed tomography.
Because patients with LS-DLBCL generally have a low risk of CNS recurrence, in the absence of clinical suspicion or a high-risk score on the CNS-International Prognostic Index (IPI), brain magnetic resonance imaging or cerebral spinal fluid sampling is not routinely required.
Epidemiology and biology
LS-DLBCL patients have similar median age (mid-sixties) and the same male predominance as DLBCL as a whole (55% men), but as expected, a higher proportion of patients have good performance status and fewer patients present with poor prognostic features such as elevated lactate dehydrogenase and systemic symptoms, likely reflecting the lower disease burden.19-27 Primary extranodal disease location is more prevalent in LS-DLBCL, representing 66% of stage I disease, with bone and stomach being the most commonly reported sites of involvement in one large patient series restricted to stage I patients.19,20,22,23,28 Racial disparities specific to LS-DLBCL are unknown.
Cell of origin (COO) analyses, by both gene expression profiling and immunohistochemistry (IHC)-based algorithms, demonstrate germinal center B-cell–like phenotype is more common in LS-DLBCL, accounting for 59% to 75% of cases compared with ∼50% in advanced-stage disease.19,24,27,29,30 Retrospective series have shown similar proportions of LS-DLBCL harbor dual protein expression of MYC and BCL2 (∼20%) and dual-rearrangement of MYC and BCL2 and/or BCL6 (∼7%) compared with all-stage DLBCL cohorts.19,30 In order to accurately classify LS-DLBCL as per the WHO criteria, phenotype by gene expression profiling or IHC and fluorescence in situ hybridization for MYC, BCL2, and BCL6 rearrangements are recommended, although do not currently impact management.
Prognosis
Patients with LS-DLBCL generally have an excellent overall survival (OS); however, several studies confirm a continuous pattern of relapse beyond 5 years, which contrasts with the rarer occurrence of late relapses in advanced-stage disease, where most patients who experience relapse do so within the first 2 years after upfront therapy.9,27 In prospective LS-DLBCL studies, the cumulative incidence of disease progression at 5 years ranges from 18% to 23% yet increases to 28% to 29% at 10 years, demonstrating the importance of longer surveillance.31 The underlying biological reason for the differential relapse pattern seen in LS-DLBCL in contrast to advanced-stage disease has not been established. Whether LS-DLBCL has a more indolent disease course manifesting as later relapse, lower rate of systemic clearance due to the higher use of localized therapies, or the possibility that these late relapses represent second malignancies have all been postulated, but more work is required to understand this phenomenon.
The IPI22 has limited utility in LS-DLBCL as most patients by definition have a favorable risk profile. In the prerituximab era, the Southwest Oncology Group (SWOG) investigators identified age >60 years, stage II disease, elevated lactate dehydrogenase (LDH), and Eastern Cooperative Oncology Group (ECOG) performance status (PS) >1 as poor-risk features and developed the stage-modified IPI ([sm-IPI], Table 2).32 Although 37% had extranodal disease, extranodal disease was not assessed as an independent risk variable. The sm-IPI separated patients into 3 risk categories and was subsequently validated in more recent series with ongoing utility.21,24,26,33,34 The NCCN-IPI, which has shown improved stratification over the IPI in patients with DLBCL, has also proven discriminatory when applied to solely LS-DLBCL cohorts (Table 2). 28,34
Outcomes of limited-stage DLBCL according to prognostic risk models
| Outcomes according to stage-modified IPI . | |||||
|---|---|---|---|---|---|
| Study design . | Population . | Treatment . | sm-IPI, n (%) . | Outcomes . | |
| Prospective studies | |||||
| Randomized phase 3 SWOG 8736, Miller (1998)32 | N = 401 | CHOP x3 + RT or CHOP x8 | 0-1 = 289 (72) 2 = 82 (20) 3 = 28 (7) 4 = 2 (<1) | 5-y PFS 0-1 = 77% 2 = 60% 3 = 34% | 5-y OS 0-1 = 82% 2 = 71% 3 = 48% |
| Prospective single-arm SWOG 0014, Persky (2008)33 | N = 60; sm-IPI ≥ 1; age > 60 | R-CHOP x3 + RT | 0 = 0 (0) 1 = 42 (70) 2 = 12 (20) 3 = 6 (10) 4 = 0 (0) | 2-y PFS 1 = 100%> 1 = 78% | |
| Prospective PET-adapted SWOG S1001, Persky et al (2019)11 | N = 132 (128 evaluable) | R-CHOP x3 Then PET− 1 x R-CHOP or PET+: RT + ibritumomab tiuxetan | 0 = 35 (27)1 = 55 (42)2 = 37 (28)3 = 5 (4) | 5-y PFS 0 = 97% 1/2 = 86% 3 = 30% | |
| Retrospective studies | |||||
| Retrospective single-center, Kumar et al (2015)24 | N = 261 (194 evaluable) | R-CHOP x3-6 +/− RT | 0 = 48 (25) 1 = 83 (43) 2 = 42 (21) 3 = 21 (11) | 5-y PFS 0 = 95.3% 1 = 87.7% 2 = 83.4% 3 = 61.9% | 5-y OS 0 = 100%, 1 = 95.2% 2 = 85.8% 3 = 74.2% |
| Retrospective multicenter, Barraclough et al (2019)19 | N = 201 (171 evaluable) | R-CHOP +/− RT | 0-1 = 93 (55) 2 = 45 (26) 3-4 = 33 (19) | 4-y OS 0-1 = 95% 2 = 86% 3-4 = 67% | |
| Retrospective multicenter, Mian et al (2014)34 | N = 1405 (254 evaluable for mIPI) | R-CHOP +/− RT | 0-1 = 127 (50) 2 = 78 (31) 3-4 = 49 (19) | 5-y OS 75% (whole cohort) HR (95% CI) 0-1 = 1.00 2 = 6.04 (2.2-16.5) 3-4 = 9.08 (3.3-25) | |
| Outcomes according to stage-modified IPI . | |||||
|---|---|---|---|---|---|
| Study design . | Population . | Treatment . | sm-IPI, n (%) . | Outcomes . | |
| Prospective studies | |||||
| Randomized phase 3 SWOG 8736, Miller (1998)32 | N = 401 | CHOP x3 + RT or CHOP x8 | 0-1 = 289 (72) 2 = 82 (20) 3 = 28 (7) 4 = 2 (<1) | 5-y PFS 0-1 = 77% 2 = 60% 3 = 34% | 5-y OS 0-1 = 82% 2 = 71% 3 = 48% |
| Prospective single-arm SWOG 0014, Persky (2008)33 | N = 60; sm-IPI ≥ 1; age > 60 | R-CHOP x3 + RT | 0 = 0 (0) 1 = 42 (70) 2 = 12 (20) 3 = 6 (10) 4 = 0 (0) | 2-y PFS 1 = 100%> 1 = 78% | |
| Prospective PET-adapted SWOG S1001, Persky et al (2019)11 | N = 132 (128 evaluable) | R-CHOP x3 Then PET− 1 x R-CHOP or PET+: RT + ibritumomab tiuxetan | 0 = 35 (27)1 = 55 (42)2 = 37 (28)3 = 5 (4) | 5-y PFS 0 = 97% 1/2 = 86% 3 = 30% | |
| Retrospective studies | |||||
| Retrospective single-center, Kumar et al (2015)24 | N = 261 (194 evaluable) | R-CHOP x3-6 +/− RT | 0 = 48 (25) 1 = 83 (43) 2 = 42 (21) 3 = 21 (11) | 5-y PFS 0 = 95.3% 1 = 87.7% 2 = 83.4% 3 = 61.9% | 5-y OS 0 = 100%, 1 = 95.2% 2 = 85.8% 3 = 74.2% |
| Retrospective multicenter, Barraclough et al (2019)19 | N = 201 (171 evaluable) | R-CHOP +/− RT | 0-1 = 93 (55) 2 = 45 (26) 3-4 = 33 (19) | 4-y OS 0-1 = 95% 2 = 86% 3-4 = 67% | |
| Retrospective multicenter, Mian et al (2014)34 | N = 1405 (254 evaluable for mIPI) | R-CHOP +/− RT | 0-1 = 127 (50) 2 = 78 (31) 3-4 = 49 (19) | 5-y OS 75% (whole cohort) HR (95% CI) 0-1 = 1.00 2 = 6.04 (2.2-16.5) 3-4 = 9.08 (3.3-25) | |
| Outcomes according to NCCN-IPI . | |||||
|---|---|---|---|---|---|
| Study design . | Population . | Treatment . | NCCN-IPI, n (%) . | Outcomes . | |
| Retrospective studies | |||||
| Retrospective multicenter Mian et al (2014)34 | N = 1405 (234 evaluable for NCCN-IPI) | (R)-CHOP +/− RT | 0-1 = 88 (38) 2-3 = 119 (51) 4-6 = 27 (11) | 5-y OS 0-1 = 98% 2-3 = 82% 4-6 = 57% | |
| Outcomes according to NCCN-IPI . | |||||
|---|---|---|---|---|---|
| Study design . | Population . | Treatment . | NCCN-IPI, n (%) . | Outcomes . | |
| Retrospective studies | |||||
| Retrospective multicenter Mian et al (2014)34 | N = 1405 (234 evaluable for NCCN-IPI) | (R)-CHOP +/− RT | 0-1 = 88 (38) 2-3 = 119 (51) 4-6 = 27 (11) | 5-y OS 0-1 = 98% 2-3 = 82% 4-6 = 57% | |
sm-IPI factors are elevated LDH, age > 60, nonbulky stage II, ECOG 2.
CI, confidence interval; HR, hazards ratio; NCCN-IPI, national comprehensive cancer network international prognostic index; PFS, progression free survival; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone.
Although extranodal disease was not included within the sm-IPI, a rigorously staged, retrospective cohort restricted to stage I disease demonstrated inferior outcomes in patients with extranodal sites compared with purely nodal disease with 10-year OS of 70% (95% CI, 58-79) vs 89% (95% CI, 74-96), respectively. This difference was observed despite similar clinical characteristics between groups, similar use of RT, and more patients with extranodal disease receiving 6 cycles of R-CHOP.20 In contrast, 2 other retrospective rituximab-era studies described similar outcomes between nodal and extranodal disease cohorts, with the caveats of small sample size and lack of PET-CT staging in the majority of patients.35,36 The prognostic value of location of extranodal disease is also controversial. In prerituximab series, primary bone and gastrointestinal disease had more favorable outcomes than nodal counterparts, yet breast, sinus, and testicular involvement have been reported to be inferior.37,38 Although 2 recent series support these findings, in a review of 141 primary extranodal LS-DLBCL, survival was not influenced by site.39-41 Based on the paucity of data overall, the prognostic significance of extranodal location in LS-DLBCL remains unclear. With the exception of primary testicular lymphoma and primary central nervous system lymphoma patients, which require separate consideration, primary extranodal disease presenting in other sites does not warrant differential management.
By definition, LS-DLBCL is nonbulky, but the cutoff for bulk has varied considerably across studies, and the prognostic impact of mass size within LS-DLBCL remains unclear. In a retrospective cohort (n = 319) from British Columbia Cancer (BC Cancer) mass size ≥5 cm predicted for PET positivity postchemotherapy (P = .008) but was not predictive of survival in multivariate analysis.21 The presence of bulk predicted for inferior event-free survival (EFS) in the large randomized Mabthera International Trial (MInT) study (HR, 1.43; 95%; CI, 1.12-1.83; P = .005), which enrolled good-prognosis young patients with all stages of DLBCL, 72% of which were early-stage disease. In this study, 45% of patients had baseline bulky disease, and all patients with stage I were required to have bulky disease to meet eligibility criteria; however, the definition of bulk ranged from >5 cm to >10 cm across institutions. All patients received 6 cycles of chemotherapy with or without rituximab, and radiotherapy was recommended by protocol for bulky disease, with 40% of patients receiving it, although not all RT was implemented according to protocol.
More recently, the prognostic impact of molecular features has been explored in dedicated LS-DLBCL cohorts. Emerging data from PET-CT–staged LS-DLBCL treated with R-CHOP–like therapy +/−RT suggests COO may not influence survival.19,24 Additionally, the presence of double expression by IHC of MYC and BCL2 or concurrent rearrangements of MYC and BCL2 and/or BCL6, so-called “double- or triple-hit” lymphoma, may not portend the same negative prognosis in patients with LS-DLBCL. However, these studies lacked sufficient power to be definitive, thus larger prospective series of routinely tested, uniformly treated populations will be required to draw firm conclusions.19,30,42
Treatment
Contemporary strategies based on R-CHOP can cure >80% of patients with LS-DLBCL.10,21,26,31 However, multiple management algorithms have evolved, and choice of treatment should take into account patient factors and risk profile. Limiting the number of cycles of chemotherapy may be desirable in patients with comorbidities and poor tolerance to systemic therapy or in patients with favorable prognostic features in whom extended chemotherapy may be unnecessary, whereas avoiding RT may be desirable depending on the location and extent of disease and concern for acute and long-term risk.
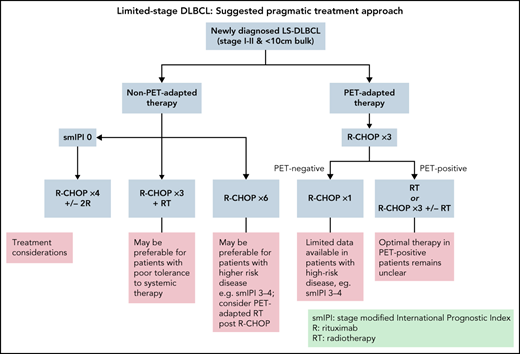
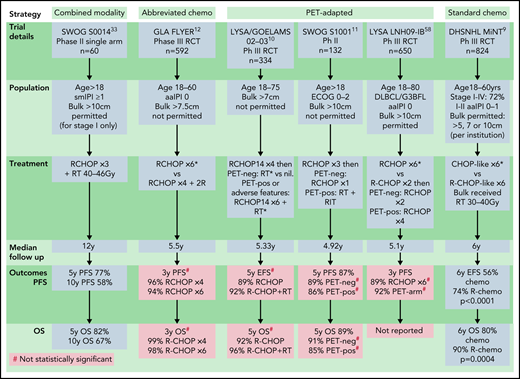
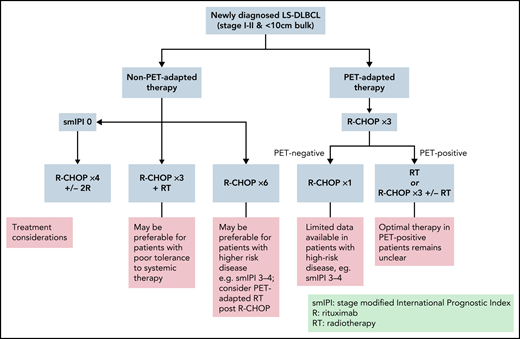
Based on rituximab-era evidence, 4 key strategies have emerged: (1) “combined modality treatment (CMT)” using abbreviated R-CHOP plus RT, (2) “standard R-CHOP” consisting of 6 cycles as per advanced-stage protocols, (3) “abbreviated R-CHOP alone” with 4 cycles of R-CHOP plus 2 additional rituximab doses, and (4) “PET-directed therapy,” most commonly with 3 to 4 cycles of R-CHOP then RT or further R-CHOP reserved for PET+ patients (Figure 2). Each has been tested in specific populations and are discussed below.
Rituximab-era prospective studies. Sm-IPI factors: elevated LDH, age > 60, nonbulky stage II, and ECOG 2. aaIPI 0: normal LDH, ECOG 0-1, stage I or II. aaIPI, age-adjusted IPI; chemo, chemotherapy; DHSNHL, German High Grade NHL study group; GLA, German Lymphoma Alliance; LYSA/GOELAMS, Lymphoma Study Association/French Acute Leukaemia and Blood Diseases West-East Group; PET-neg, negative PET scan; PET-pos, positive PET scan; RCT, randomized control trial. *Control arm of study. #Difference is not statistically significant.
Rituximab-era prospective studies. Sm-IPI factors: elevated LDH, age > 60, nonbulky stage II, and ECOG 2. aaIPI 0: normal LDH, ECOG 0-1, stage I or II. aaIPI, age-adjusted IPI; chemo, chemotherapy; DHSNHL, German High Grade NHL study group; GLA, German Lymphoma Alliance; LYSA/GOELAMS, Lymphoma Study Association/French Acute Leukaemia and Blood Diseases West-East Group; PET-neg, negative PET scan; PET-pos, positive PET scan; RCT, randomized control trial. *Control arm of study. #Difference is not statistically significant.
Combined modality treatment (abbreviated chemotherapy plus RT)
Early studies of RT alone for localized aggressive lymphoma demonstrated curative potential, but relapses outside the RT field were common, leading to the incorporation of chemotherapy.43,44 In the prerituximab era, abbreviated CHOP (3 cycles) plus RT became the standard of care when the initial report of the phase III SWOG S8736 study demonstrated improved PFS and OS compared with 8 cycles of CHOP alone.32 With a median 4.2 years of follow-up, CHOP plus RT yielded a 5-year OS of 82% vs 72% with 8 cycles of CHOP (hazard ratio [HR], 1.7; 95% CI, 1.1-2.7). However, longer follow-up revealed more delayed relapses, along with late RT complications in the combined modality arm, negating the survival advantage.31,32
Although no randomized studies have assessed the benefit of rituximab with combined modality therapy exclusively in LS-DLBCL, available data suggest benefit. 9,33 The single-arm phase 2 SWOG S0014 study evaluated 3 cycles of R-CHOP plus 40 to 46 Gray (Gy) RT in LS-DLBCL patients with at least 1 adverse prognostic feature according to the sm-IPI.33 The 5- and 10-year OS were 82% and 67%, respectively,31 which appeared to be better than the historical SWOG cohort,32 despite the inclusion of patients with higher risk features. Interestingly, the addition of rituximab did not mitigate the risk of delayed relapses.33 Although 3 cycles of R-CHOP and RT remains an acceptable approach for LS-DLBCL and may be desirable for some patients, the lack of survival benefit and delayed toxicities observed with extended follow-up of SWOG S8736, along with improved outcomes following the addition of rituximab, has called into question the need for universal RT for all patients with LS-DLBCL.
More recently, the use of modern RT technologies, reduced RT fields, and lower RT doses have refined radiation delivery, likely reducing off-target organ toxicity while maintaining efficacy.45-47 However, the risk of delayed toxicities associated with newer techniques cannot be assessed without longer follow-up. In patients with poor chemotherapy tolerance, routine RT may be chemotherapy sparing, and thus CMT remains an important strategy for some patients. However, with recent data exploring abbreviated R-CHOP alone in good-risk patients and newer PET-adapted strategies as discussed below, the routine use of CMT is no longer recommended in all patients, and close consultation with radiation oncology in the decision to include RT is essential in order to evaluate the specific acute and long-term risks of RT exposure.
Standard R-CHOP (6 cycles)
The randomized phase III MInT study evaluated the benefit of rituximab when added to 6 cycles of R-CHOP–like chemotherapy in DLBCL patients aged 18 to 60 with favorable prognosis (aaIPI 0-1), demonstrating an OS advantage in rituximab-treated patients. Although this trial did not exclusively enroll LS-DLBCL, 72% of patients had stages I to II disease, and only 3% had a baseline mass >10 cm. Six-year OS was 90% with R-chemotherapy compared with 80% with chemotherapy alone. Outcomes were particularly favorable in patients with mass size <5 cm and no other IPI risk factors, with a 6-year OS of 95% (Figure 2).9 Although the MInT trial recommended RT for bulky disease, only 41% of patients in the rituximab arm received it, and outcomes were excellent with R-CHOP alone in nonbulky patients.
A retrospective Japanese single-institution series of 190 LS-DLBCL patients across all risk groups reported results with uniformly prescribed 6 cycles of R-CHOP. The authors claimed a plateau in relapses and 5-year OS was 90%; however, median follow-up was only 52 months.25 Several additional retrospective analyses have suggested equivalent efficacy of full-course R-CHOP compared with CMT, enabling the avoidance of RT with the increased systemic therapy as described above (Table 3).24,48,49
Rituximab-era retrospective studies
| Therapeutic strategy . | Study design . | n . | Patient characteristics . | Treatment details . | Median follow-up (y) . | PFS . | OS (95% CI) . |
|---|---|---|---|---|---|---|---|
| Combined modality vs standard R-CHOP | Multicenter Osaka Lymphoma Study Group48 | 137 | All LS-DLBCL Nonbulky only (<10 cm) Details not specified | R-CHOP 3-4 + RT vs R-CHOP 6-8 | 2.83 | 3-y PFS* 74% R-CHOP vs 89.7% R-CHOP + IFRT | 3-y OS* 85.5% R-CHOP vs 96.2% R-CHOP+IFRT |
| Combined modality vs standard R-CHOP | Single-center Memorial-Sloan Kettering24 | 261 | All LS-DLBCL Nonbulky only (<10 cm) Age: all (>60 = 48%) stage II: 32% Extranodal: 51% Bulk: 0% (excluded) | R-CHOP ×3-4 R-CHOP 3-4 + RT R-CHOP ×6 R-CHOP ×6 + RT | 4.7 | 5-y PFS 92% 94% R-CHOP ×3-4 89% R-CHOP x3 + IFRT 79.9% R-CHOP x6 89.8% R-CHOP x6 + IFRT | 5-y OS 93% 100% R-CHOP ×3-4 93.5% R-CHOP + RT 84.5% R-CHOPx6 97.8% R-CHOPx6 + RT |
| Combined modality vs standard R-CHOP | Multicenter SEER Database49 | 874 | Elderly LS-DLBCL Bulk status not reported Age: ≥66 y Stage II: 40% B Symptoms: 11% Extranodal: 33% | R-CHOP ×3-4 + RT vs R-CHOP ×6-8 | 4.2 | 5-y EFS 24% R-CHOP ×3-4 + IFRT vs 33% R-CHOP ×6-8 HR 0.71 P = .02 | 5-y OS* 77% R-CHOP ×3-4 + RT vs 76% R-CHOP ×6-8 |
| Standard chemo +/− IFRT | Single-center MD Anderson7 | 469 (190 LS- DLBCL) | Stage I-IV included Bulk permitted: defined as >5 cm Age: all (medial 61 y) Stage I/II: 20%/20% Other parameters not specifically reported for LS-DLBCL | R-CHOP-like 6-8 +/− RT 30% “other” chemo | 3 | LS-DLBCL cohort results: 5-y PFS 81% overall 82% RT vs 68% no RT (P = .003) | LS-DLBCL cohort results: 5-y OS 83% overall 92% RT vs 73% no RT (P = .007) |
| Standard R-CHOP +/− RT | Multicenter NCCN Institutions51 | 841 (402 LS-DLBCL) | Stages I-IV included Bulk permitted: Defined as ≥10 cm Age: all (median 57 y) Stage I/II: 26%/22% B Symptoms: 28.5% Extranodal: 26% Bulk: 23% | R-CHOP ×6-8 +/− IFRT | 4.5 | LS-DLBCL cohort results: HR 1.81, P = .15 for RT | LS-DLBCL cohort results: OS HR 0.94, P = .89 for RT |
| Standard R-CHOP +/− RT | Single-center Seoul National University Hospital50 | 198 | All LS-DLBCL Bulk permitted: defined as ≥7 cm Age: Median 55 Stage II: 67% PS > 1: 7.6% Elevated LDH: 41% B Symptoms: 14% Extranodal: 63% Bulk: 30% | R-CHOP ×6-8 +/− IFRT | 3.3 | 3-y PFS 85.8% 83.9% no RT vs 92.7% RT P = .021 | 3-y OS 88.9% 95% no RT vs 87.1% RT P = .014 |
| Combined modality vs abbreviated or standard R-CHOP alone | Single-center Memorial Sloan-Kettering20 | 341 | Stage I only Nonbulky only (≤7.5 cm) Age: Median 60 y Stage II: 0% PS > 1: 3% Elevated LDH 22% B Symptoms: 3% Extranodal: 66% Bulk: 0% (excluded) | R-CHOP ×3-4 +/− RT R-CHOP ×6 +/− RT | 5.5 | 10-y PFS 77% 63% Extranodal vs 85% Nodal HR 0.35 for extranodal disease in favor of RT P < .001 | 5-y OS 94% 10-y OS 77% 70% extranodal vs 89% Nodal HR 0.26 for extranodal disease in favor of RT P < 0.001 |
| PET-adapted | Single-center BC Cancer21 | 319 | All LS-DLBCL Nonbulky only (<10 cm) No B Symptoms Age: median 68 y Stage II: 8% PS > 1: 13% B symptoms: 0% Elevated LDH: 52% Extranodal: 37% Bulk: 0% (excluded) PE+: 18% | R-CHOP x3 then PET+: RT PET−: R-CHOP ×1 | 6.25 | 5-y PFS 84% 88% PET−* vs 74% PET+* | 5-y OS 87% 90% PET−* vs 77% PET+* |
| Therapeutic strategy . | Study design . | n . | Patient characteristics . | Treatment details . | Median follow-up (y) . | PFS . | OS (95% CI) . |
|---|---|---|---|---|---|---|---|
| Combined modality vs standard R-CHOP | Multicenter Osaka Lymphoma Study Group48 | 137 | All LS-DLBCL Nonbulky only (<10 cm) Details not specified | R-CHOP 3-4 + RT vs R-CHOP 6-8 | 2.83 | 3-y PFS* 74% R-CHOP vs 89.7% R-CHOP + IFRT | 3-y OS* 85.5% R-CHOP vs 96.2% R-CHOP+IFRT |
| Combined modality vs standard R-CHOP | Single-center Memorial-Sloan Kettering24 | 261 | All LS-DLBCL Nonbulky only (<10 cm) Age: all (>60 = 48%) stage II: 32% Extranodal: 51% Bulk: 0% (excluded) | R-CHOP ×3-4 R-CHOP 3-4 + RT R-CHOP ×6 R-CHOP ×6 + RT | 4.7 | 5-y PFS 92% 94% R-CHOP ×3-4 89% R-CHOP x3 + IFRT 79.9% R-CHOP x6 89.8% R-CHOP x6 + IFRT | 5-y OS 93% 100% R-CHOP ×3-4 93.5% R-CHOP + RT 84.5% R-CHOPx6 97.8% R-CHOPx6 + RT |
| Combined modality vs standard R-CHOP | Multicenter SEER Database49 | 874 | Elderly LS-DLBCL Bulk status not reported Age: ≥66 y Stage II: 40% B Symptoms: 11% Extranodal: 33% | R-CHOP ×3-4 + RT vs R-CHOP ×6-8 | 4.2 | 5-y EFS 24% R-CHOP ×3-4 + IFRT vs 33% R-CHOP ×6-8 HR 0.71 P = .02 | 5-y OS* 77% R-CHOP ×3-4 + RT vs 76% R-CHOP ×6-8 |
| Standard chemo +/− IFRT | Single-center MD Anderson7 | 469 (190 LS- DLBCL) | Stage I-IV included Bulk permitted: defined as >5 cm Age: all (medial 61 y) Stage I/II: 20%/20% Other parameters not specifically reported for LS-DLBCL | R-CHOP-like 6-8 +/− RT 30% “other” chemo | 3 | LS-DLBCL cohort results: 5-y PFS 81% overall 82% RT vs 68% no RT (P = .003) | LS-DLBCL cohort results: 5-y OS 83% overall 92% RT vs 73% no RT (P = .007) |
| Standard R-CHOP +/− RT | Multicenter NCCN Institutions51 | 841 (402 LS-DLBCL) | Stages I-IV included Bulk permitted: Defined as ≥10 cm Age: all (median 57 y) Stage I/II: 26%/22% B Symptoms: 28.5% Extranodal: 26% Bulk: 23% | R-CHOP ×6-8 +/− IFRT | 4.5 | LS-DLBCL cohort results: HR 1.81, P = .15 for RT | LS-DLBCL cohort results: OS HR 0.94, P = .89 for RT |
| Standard R-CHOP +/− RT | Single-center Seoul National University Hospital50 | 198 | All LS-DLBCL Bulk permitted: defined as ≥7 cm Age: Median 55 Stage II: 67% PS > 1: 7.6% Elevated LDH: 41% B Symptoms: 14% Extranodal: 63% Bulk: 30% | R-CHOP ×6-8 +/− IFRT | 3.3 | 3-y PFS 85.8% 83.9% no RT vs 92.7% RT P = .021 | 3-y OS 88.9% 95% no RT vs 87.1% RT P = .014 |
| Combined modality vs abbreviated or standard R-CHOP alone | Single-center Memorial Sloan-Kettering20 | 341 | Stage I only Nonbulky only (≤7.5 cm) Age: Median 60 y Stage II: 0% PS > 1: 3% Elevated LDH 22% B Symptoms: 3% Extranodal: 66% Bulk: 0% (excluded) | R-CHOP ×3-4 +/− RT R-CHOP ×6 +/− RT | 5.5 | 10-y PFS 77% 63% Extranodal vs 85% Nodal HR 0.35 for extranodal disease in favor of RT P < .001 | 5-y OS 94% 10-y OS 77% 70% extranodal vs 89% Nodal HR 0.26 for extranodal disease in favor of RT P < 0.001 |
| PET-adapted | Single-center BC Cancer21 | 319 | All LS-DLBCL Nonbulky only (<10 cm) No B Symptoms Age: median 68 y Stage II: 8% PS > 1: 13% B symptoms: 0% Elevated LDH: 52% Extranodal: 37% Bulk: 0% (excluded) PE+: 18% | R-CHOP x3 then PET+: RT PET−: R-CHOP ×1 | 6.25 | 5-y PFS 84% 88% PET−* vs 74% PET+* | 5-y OS 87% 90% PET−* vs 77% PET+* |
IFRT, involved field radiotherapy.
Not statistically significant.
The additional benefit of RT following full-course R-CHOP in LS-DLBCL remains uncertain. Retrospective data are conflicting. Superior PFS and OS were seen with the addition of RT to 6 to 8 cycles of R-CHOP in LS-DLBCL patients in 2 studies7,50 but no difference in LS-DLBCL in another.51 In one study restricted to stage I disease, a subgroup analysis of patients with extranodal disease suggested a benefit of adding RT to R-CHOP with a HR 0.35 and 0.26 for PFS and OS, respectively, in favor of RT and a median follow-up of 5.5 years.20 However, a mixture of R-CHOP 3 to 4 cycles and 6 to 8 cycles was used, with imbalances in patient characteristics across the 4 groups (Table 3). Taken together, the data suggest that the benefit of routine RT after full-course R-CHOP is small at best and may reflect patient selection bias but also does not account for potential late radiation effects, often not detected for decades after therapy.
Based on available data, 6 cycles of R-CHOP alone has become an acceptable treatment of LS-DLBCL. It has been particularly favored for patients with high-risk LS-DLBCL, as these patients have been under-represented in CMT studies. Similarly, it enables the avoidance of late toxicity of RT, which is desirable in certain patients, such as young females with large volumes of mediastinal or breast tissue in the radiation field. However, in many cases, 6 cycles of R-CHOP may represent over-treatment, making abbreviated R-CHOP alone or PET-tailored therapy desirable as discussed below.
Abbreviated R-CHOP alone
In patients aged 18 to 60 years, with no adverse features (normal LDH, PS 0-1, tumor diameter <7.5 cm), the landmark phase III “FLYER” study demonstrated noninferior 3-year PFS with 4 cycles of R-CHOP plus 2 additional rituximab doses compared with 6 cycles of R-CHOP (96% vs 94%) and considerably lower toxicity with abbreviated R-CHOP.12 This has created a new standard for young, good-prognosis LS-DLBCL; however, follow-up is relatively short given the established ongoing relapse risk beyond 5 years. The necessity for 2 additional doses of rituximab following 4 cycles of R-CHOP is uncertain and has not been administered in PET-based trials. Importantly, interim PET scanning was not used in this study, hence outcomes specifically in those who may have been PET+ after 2 to 4 cycles is unknown. Additionally, uncertainty remains regarding generalizability of these findings to elderly patients or those with high-risk features (Figure 2).
PET-adapted therapy
The spectrum of LS-DLBCL outcomes according to risk profile, coupled with concerns regarding toxicity of RT or full-course chemotherapy, has led to evaluation of PET-adapted delivery of RT or tailoring the number of chemotherapy cycles in order to limit toxicity in patients with chemotherapy-sensitive disease.
The prospective LYSA/French Acute Leukaemia and Blood Diseases West-East Group 02-03 trial randomized patients with nonbulky (<7 cm) LS-DLBCL who achieved a complete response by PET scan (PET-CR) after 4 cycles of 14-day interval R-CHOP (R-CHOP14) to 40 Gy RT or observation.10 PET-CR patients with no adverse prognostic features received only 4 cycles of chemotherapy, whereas patients with ≥1 adverse feature (sm-IPI > 0) received 6 cycles of R-CHOP14. Patients achieving only a partial response (PR) on interim PET received 6 cycles of R-CHOP14 plus RT. In the PET-CR group (n = 281), the primary endpoint of 5-year EFS was similar between the RT and non-RT arms, but 44% received 6 cycles of R-CHOP14 due to adverse risk factors; hence, the study could not assess the role of PET-adapted abbreviated R-CHOP in higher-risk cohorts. These results support the use of abbreviated R-CHOP alone in patients with no adverse risk factors who achieve a CR after 4 cycles and the omission of radiation therapy after 6 cycles of R-CHOP in higher-risk patients achieving an interim PET-CR. Although this study used the dose-dense regimen R-CHOP14, given the excellent outcomes of patients in studies using R-CHOP 21 and the demonstration of comparability in head-to-head trials,27,52 this is not likely warranted (Figure 2).
The phase II SWOG S1001 study evaluated PET-tailored therapy in patients with nonbulky (<10 cm) LS-DLBCL. Interim PET scanning was performed after 3 cycles of R-CHOP in 128 patients, of which 89% were considered PET− and were planned for 1 further cycle of R-CHOP, whereas PET+ patients were intended to receive 36 to 45 Gy IFRT plus 90Y ibritumomab tiuxetan26 Patients with adverse risk factors were permitted in this trial, with sm-IPI >0 in 73% of patients. Five-year OS for the whole cohort was 89%, with no statistical difference between PET+ and PET− groups (85% vs 91%, respectively). This trial supports the use of 4 cycles of R-CHOP alone in patients with LS-DLBCL achieving a negative interim PET scan. Due to the nonrandomized nature of this study and the small number of PET+ patients, the role of radioimmunotherapy in addition to IFRT in PET+ patients cannot be assessed. Additionally, although higher-risk patients were enrolled in this trial, the sm-IPI demonstrated inferior outcomes in these patients, and the effect of omitting RT in those with adverse risk features achieving interim PET negativity cannot be firmly established (Figure 2).
The largest study to evaluate uniformly applied abbreviated R-CHOP with PET-directed RT was the retrospective BC Cancer experience of 319 patients with nonbulky (<10 cm) LS-DLBCL.21 All patients received 3 cycles of R-CHOP, then underwent PET. PET− patients (n = 254) received 1 additional cycle of R-CHOP, whereas 59 (18%) PET+ patients received involved-site RT. The 5-year OS was 87%. Importantly, PET− patients achieved a 90% 5-year OS with 4 cycles of R-CHOP and were spared RT, yet the 5-year OS of PET+ patients was only 77% despite the addition of RT, indicating that optimal therapy for this group requires further evaluation. Of note, PET status after 3 cycles of R-CHOP was predictive of time-to-progression (5-year TTP 92% for PET− and 80% for PET+ patients, P = .004) but not survival (Table 3).
The largest phase III trial performed in LS-DLBCL to date, LYSA LNH09-1B, randomized 650 good-risk (aaIPI = 0) LS-DLBCL patients aged 18 to 80 years to either 6 cycles of R-CHOP or a PET-adapted strategy.53 The experimental arm underwent PET after 2 cycles of R-CHOP (PET2) and PET+ patients continued to 6 cycles of R-CHOP, whereas the PET− group received only 2 further R-CHOP cycles (total of 4 cycles). Reported in abstract form, with 5.1 years of follow-up, the 3-year PFS was 89% (95% CI, 85.3-92.2) in the standard R-CHOP arm and 92.0% (95% CI, 88.3-94.5) in the PET-adapted arm, demonstrating noninferiority (HR, 0.724; 90% CI, 0.504-1.040). In the experimental arm, 80.4% of patients had a negative PET2, receiving only 4 cycles of R-CHOP. In the entire study cohort, outcomes in patients with PET2 positivity were not inferior to PET2− patients; however, patients with a positive PET after 4 cycles (“PET4”) had an inferior PFS (P < .001). Of note, patients with PET4 positivity in either arm were taken off study and treatment of these patients not reported. In contrast to the FLYER study, which only recruited patients aged ≤60 years, 44% of the LNH09-1B cohort were older than 59 years. Although further details and longer follow-up are needed, these preliminary results demonstrate that abbreviated R-CHOP is sufficient in the absence of adverse features in patients with a negative interim PET, regardless of age.
Compiled evidence suggests that favorable-risk patients with LS-DLBCL achieving PET negativity after 2 to 4 cycles of R-CHOP have an excellent outcome with 4 cycles of R-CHOP alone. However, optimal management in patients with a positive interim PET scan remains unclear, and IFRT and/or additional cycles of chemotherapy should be considered. Due to the limited number of patients with high-risk features assessed in available trials, the value of this approach in patients with adverse prognostic factors remains less clear. However, inclusion of interim PET after 2 to 4 cycles of therapy is a valuable prognostic tool and informs treatment in those patients planned for PET-directed treatment, so it is recommended where available.
Other considerations
Elderly patients
Elderly patients comprise a distinct proportion of LS-DLBCL. Older patients have poorer outcomes related to factors other than age alone, and many are not fit for full dose R-CHOP. Limited data exist specifically for unfit or very elderly patients with LS-DLBCL. The French Groupe d'Etude des Lymphomes de l'Adulte nonrandomized phase II study that evaluated 6 cycles of R-miniCHOP in patients aged >80 years comprised 25% stage I to II disease with a 2-year OS of 68.5% reported for these patients; however, no additional details of the risk features or outcomes of this group were published specifically.54 Planned treatment with combined modality therapy may be a desirable approach in elderly patients as it minimizes the number of chemotherapy cycles while introducing the added benefit of RT, of which late effects are less of a concern in this group due to the overall life expectancy. Of note, in the prerituximab era, the phase III Groupe d'Etude des Lymphomes de l'Adulte study in patients aged >60 years demonstrated no benefit of the addition of RT to 4 cycles of CHOP in good-prognosis stage I to II disease, potentially supporting the body of evidence for abbreviated chemotherapy alone in this group; however, patients all received full-does CHOP, and PET staging was not performed.55 Alternatively, PET-adapted approaches may also be appropriate, with abbreviated R-CHOP alone demonstrated effective in patients up to age 80 years with an aaIPI of 0 with a negative interim PET and PET-directed RT data applicable to all ages.21,26,53 Front-line therapy decisions should consider the limited ability for salvage of relapsed disease with high-dose treatment and transplantation.
Central nervous system prophylaxis
The role of high-dose methotrexate as prophylaxis against CNS relapse remains controversial in advanced disease, and data are virtually nonexistent in LS-DLBCL. Sites strongly associated with risk of CNS disease such as primary testicular lymphoma or renal/adrenal involvement may warrant this approach, but data specific to LS-DLBCL involving other sites or with high-risk features are not available; thus, CNS prophylaxis is not routinely recommended for these latter scenarios.56-60
Completely resected disease
Diagnostic excisional biopsy can occasionally lead to removal of all visible disease. Although patients with resected disease have been included in some studies, outcomes are not well described. Historical data suggest that localized therapies such as surgery or RT alone cannot prevent systemic relapses and thus are not favored.43 Standard R-CHOP, combined modality therapy or abbreviated R-CHOP alone may all be used. The latter has been tested in a dedicated phase II study of 23 patients, with only 1 relapse observed and 5-year OS of 95%.61
Relapsed disease
For LS-DLBCL patients who experience subsequent relapse, disease treatment algorithms should mirror those of relapsed advanced-stage disease and therapeutic decisions should consider the initial treatment received, disease-free interval, underlying biology of the disease, and patient fitness for intensive therapies such as high-dose therapy and stem cell transplantation. To date, no novel therapy studies have exclusively targeted patients with relapse following curative-intent therapy for LS-DLBCL.
Posttreatment follow-up
After therapy completion, for patients achieving complete response, there are no explicit monitoring guidelines for patients with LS-DLBCL.4,62,63 General recommendations within the Lugano classification for DLBCL suggest follow-up intervals of every 3 months within the first 2 years, every 6 months for an additional 3 years, then annually thereafter to monitor for late relapse and treatment-related adverse events.13 Given the distinct continuous relapse risk in LS-DLBCL, patients should be educated regarding the need for longer-term follow-up. The value of routine surveillance imaging has not been demonstrated and therefore is generally discouraged.13
Conclusion and future directions
LS-DLBCL long-term disease-free survival in the majority of patients is in excess of 80%, regardless of treatment approach. 9,21,31 Recent studies evaluating optimization of therapy for this unique subgroup of DLBCL have led to new management algorithms. Options include combined modality therapy, full-course R-CHOP, abbreviated chemotherapy alone, and PET-adapted approaches. Therapeutic decisions must balance risk of disease recurrence and feasibility of treatment delivery due to patient factors (Figure 3). Treatment selection should consider both chemotherapy toxicity such as cardiac complications, myelosuppression, infection, and neuropathy, as well as delayed effects of RT including second malignancies and organ compromise. Improved supportive care for chemotherapy and newer RT dosing techniques may minimize sequelae, but patient comorbidities, location of disease, and patient preferences will remain relevant factors.
In young patients (<60 years) with no adverse prognostic features, R-CHOP times 4 (with or without 2 additional doses of rituximab) is a reasonable option. For remaining patients, PET-adapted therapy may enable risk stratification and is likely to be central to future paradigms. Patients with a negative interim PET scan may be appropriately treated with abbreviated R-CHOP alone, although management of patients with a positive interim PET is less clear. Similarly, management of patients with adverse prognostics features warrants further evaluation as these patients have been under-represented in most LS-DLBCL trials.
As the molecular subtyping of DLBCL evolves, it is likely to affect prognostication and decision making in LS-DLBCL; however, due to the unique clinical features, molecular studies dedicated to LS-DLBCL are desperately needed. Utility of novel targeted therapies based on strong biological rationale in advanced-stage disease may be relevant to LS-DLBCL; however, given the excellent outcomes from current treatments, careful consideration of any additional toxicity is needed, and risk stratification needs refinement in order to identify those in greatest need. Future efforts to tailor management should focus on improved risk stratification in order to minimize toxicity in patients with good-prognosis disease while improving outcomes in poorer-risk patients. Refining PET-adapted approaches using novel PET metrics coupled with enhanced risk stratification (using molecular testing combined with clinical prognostication) may improve both prognostic assessment accuracy and adaptive management strategies, yielding better outcomes in the future.
Authorship
Contribution: All authors contributed to writing the manuscript and approved the final submitted version.
Conflict-of-interest disclosure: E.A.H.: Research funding to institution: Bristol Myers Squibb/Celgene, Merck KgA, Astra Zeneca, and F. Hoffmann-La Roche. Advisory board: F. Hoffmann-La Roche,* Antigene,* Bristol Myers Squibb, Astra Zeneca, Novartis,* Merck Sharpe Dohme,* and Gilead* (*paid to institution). Speaker engagement: Roche (institution), Astra Zeneca (institution), Janssen, and Regeneron. Consultancy: Specialized therapeutics. A.B.: Conference sponsorship and speakers fees: Roche. L.H.S.: Speaker engagement: AbbVie, Astra Zeneca, Bristol Myers Squibb, F. Hoffmann-La Roche, Gilead, Janssen, and Sandoz. Consultancy: Abbvie, Astra Zeneca, Bristol Myers Squibb, F. Hoffmann-La Roche, Incyte, and Janssen.
Correspondence: Eliza A. Hawkes, Department of Oncology, ONJ Building, Austin Health, PO Box 5555, Heidelberg, VIC 3084, Australia; e-mail: eliza.hawkes@onjcri.org.au.
Send data sharing requests via e-mail to the corresponding author.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal