In this issue of Blood, Funk et al1 investigate translational strategies to improve efficacy of chimeric antigen receptor T (CART) cells for chronic lymphocytic leukemia (CLL). They found that addition of duvelisib, a phosphatidylinositol 3-kinase δ/γ (PI3Kδ/γ) inhibitor, during manufacturing of CART cells from patients with CLL results in a CAR-T product (duv-CAR) with stem-like qualities and improved metabolic fitness, resulting in enhanced efficacy (see figure). The authors demonstrate that these favorable alterations after in vitro treatment with duvelisib are caused by epigenetic reprogramming.
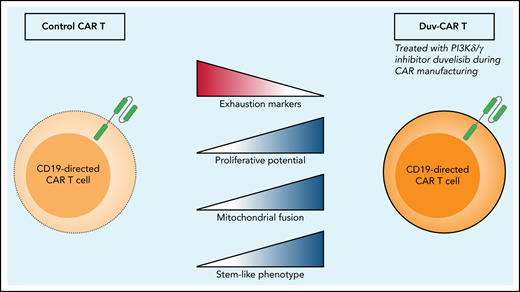
When Funk et al compare control CART cells with CART cells that have been treated with the PI3Kδ/γ inhibitor duvelisib (eg, duv-CAR), they find increased levels of exhaustion markers TIM-3 and LAG-3 in the control CART cells and increased proliferation, mitochondrial fusion proteins MFN1 and MFN2, and a more stem-like phenotype of the duv-CAR.
When Funk et al compare control CART cells with CART cells that have been treated with the PI3Kδ/γ inhibitor duvelisib (eg, duv-CAR), they find increased levels of exhaustion markers TIM-3 and LAG-3 in the control CART cells and increased proliferation, mitochondrial fusion proteins MFN1 and MFN2, and a more stem-like phenotype of the duv-CAR.
The authors address an important issue of the limited success of CART cell therapy in CLL, likely because of acquired T-cell dysfunction.2 In CLL, autologous T-cell therapies yield disappointing response rates, especially compared with their activity in more aggressive lymphoproliferative diseases (eg, acute lymphocytic leukemia). Acquired T-cell dysfunction that progresses during the course of the disease includes altered cytokine secretion profiles, expression of exhaustion markers, and reduced cytotoxicity of CD8+ T cells, in addition to skewing toward an effector memory phenotype. These observations correlate with findings of reduced metabolic plasticity of CD8+ T cells in CLL.3 Such CD8+ T cells have lower reserves of the glucose transporter GLUT1 and exhibit a skewed mitochondrial metabolic profile, which is maintained even after activation of T cells. The clinical implications of these findings were demonstrated by enhanced mitochondrial biogenesis of CD19-directed CAR+CD8+ T cells in infusion products of patients with CLL that show a complete response compared with nonresponders. Mitochondrial biogenesis correlated with the persistence of these cells after infusion and with clinical outcome.3 With this knowledge in mind, Funk et al set out to improve the CART cell phenotype and metabolic function during the manufacturing process, an approach that is gaining support.
It is becoming clear that instead of effector cells with a high cytotoxic potential, less differentiated phenotypes including memory and stem-like cells produce more favorable outcomes after CART cell therapy. These cell types are more persistent and undergo extensive expansion in vivo, recently confirmed for B-cell acute lymphoblastic leukemia in an excellent study by Chen et al,4 reporting the largest clinically annotated molecular database in CART cell therapy to date. In CLL, Fraietta et al5 were able to show that the frequency of CD45RO−CD27+CD8+ cells in the leukapheresis product could serve as a biomarker for response. The patient’s initial T-cell subset composition is determined by age and disease history and therefore an uncontrolled element. The study by Funk et al is particularly relevant as CAR manufacturing provides a window of opportunity to enhance numbers of stem cell memory, naïve, and memory T-cell phenotypes by in vitro treatment with duvelisib. Because the cellular differentiation state is strongly epigenetically regulated, but largely unexplored in CLL T cells,6 the authors draw a reasonable conclusion that epigenetic reprogramming by PI3Kδ/γ inhibition leads to more stem-like properties of the CART cells.
The authors describe an increase in protein levels of mitochondrial fusion proteins MFN1 and MFN2 and quantification of mitochondrial content by transmission electron microscopy, indicating that, in duv-CAR, mitochondrial content in relation to cell surface is increased. However, Seahorse analysis reveals no difference in oxygen consumption rate (OCR) between control CAR and duv-CAR, a rather counterintuitive finding. The question therefore remains whether the suggested increased mitochondrial fusion, which based on previous research should lead to more efficient oxidative phosphorylation, functionally alters the metabolism of the CART cells generated in this study. A possible explanation is provided by Yu et al,7 where they show in a melanoma tumor model that tumor-infiltrating lymphocytes (TILs) have higher mitochondrial content than splenic T cells, which was confirmed by electron microscopy. However, the quality of the individual mitochondria was compromised in TILs, as shown by depolarization and damaged membrane structures.7 Thus, increased mitochondrial content does not by definition means increased mitochondrial fitness, and future research effort should be devoted to characterizing the metabolic differences within the duv-CAR T cells and their role in improved persistence and functionality in vivo.
Strategies using in vitro treatment with epigenetic modulators such as decitabine and now PI3Kδ/γ inhibition show encouraging results and might be explored in future clinical studies.8 However, the question that remains now is how stable epigenetic changes induced during CAR manufacturing are in vivo. Although the authors show enhanced expansion and persistence of their duv-CAR for several weeks in a murine model system, the percentage of CLL cells in that model is not nearly as high as it is in patients. It would be helpful to compare the transcriptome and epigenome of the preinfusion product and of CART cells at several time points after infusion to assess persistence and stability of the features induced during manufacturing. A possible advantage of a small molecule such as duvelisib, which also has clinical activity against the CLL clone, is that the drug can be given at intervals after CART cell infusion if there are indications of early molecular relapse.
Despite the great recent successes in CLL treatment, most patients will sooner or later develop disease resistant to currently available treatments. Therefore, alternative treatments such as autologous cellular treatment strategies are required. Therefore, what options do we now have to improve CART cell efficacy for CLL that can be tested in clinical trials? One possibility that follows from the paper of Funk et al is modulation of the product during manufacturing. Another option is to administer small molecules concurrently with CD19 CART cell immunotherapy as is being tested for ibrutinib.9 A third approach could be to use autologous T cell–based therapies such as CART cells as consolidation to eradicate residual disease after potent induction treatment with agents that do not negatively affect T-cell viability. Our observation of T-cell recovery after elimination of leukemic cells by venetoclax treatment is an indication of the feasibility of such an approach.10 The strategy (or combination of strategies) that will prove to be most vital is currently unclear, but translational studies such as that of Funk et al do provide clinically testable options.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal