Key Points
[68Ga]Pentixafor is a novel PET tracer that targets the chemokine receptor CXCR4, which is overexpressed in MALT lymphoma.
In gastric MALT lymphoma after H pylori eradication, [68Ga]Pentixafor–PET shows high accuracy for detection of residual disease.
Abstract
Posttreatment evaluation of gastric mucosa-associated lymphoid tissue (MALT) lymphoma currently relies on esophagogastroduodenoscopy with histological assessment of biopsies. Overexpression of the G protein–coupled C-X-C chemokine receptor type 4 (CXCR4) has been previously observed in MALT lymphoma. The aim of this prospective study was to evaluate positron emission tomography (PET) with the novel CXCR4 tracer [68Ga]Pentixafor as a potential alternative to follow up biopsies for assessment of residual disease (noncomplete remission [CR]) after first-line Helicobacter pylori eradication. Forty-six post–H pylori eradication [68Ga]Pentixafor–PET/magnetic resonance imaging (MRI) examinations of 26 gastric MALT lymphoma patients, and 20 [68Ga]Pentixafor–PET/MRI examinations of 20 control group patients without lymphoma, were analyzed. In the MALT lymphoma group, time-matched gastric biopsies were used as reference standard and showed CR in 6 cases. Pooled examination-based accuracy, sensitivity, specificity, and positive and negative predictive values of [68Ga]Pentixafor–PET for detection of residual gastric MALT lymphoma at follow-up were 97.0%, 95.0%, 100.0%, 100.0%, and 92.9%, respectively. Maximum and mean PET standardized uptake values showed moderate correlation with immunohistochemistry-based CXCR4+ cell counts, with correlation coefficients of r = 0.51 and r = 0.52 (P = .008 and P = .006). In summary, CXCR4 imaging with [68Ga]Pentixafor–PET may represent a promising test for assessment of residual gastric MALT lymphomas after H pylori eradication.
Introduction
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) accounts for 7% to 8% of newly diagnosed lymphomas; the stomach is its most common organ of origin (30% to 50% of all cases).1 Because gastric MALT lymphoma is closely associated with Helicobacter pylori infection, antibiotic H pylori eradication is the first-line treatment; refractory cases receive second-line radiation or systemic chemo- or immunotherapy.1,2 Contrary to the majority of lymphoma subtypes that rely heavily on positron emission tomography (PET) with 2-deoxy-2-[18F]fluoro-d-glucose to assess disease and treatment response,3,4 the reference test for gastric MALT lymphoma is histological assessment of multiple biopsies taken by esophagogastroduodenoscopy according to GELA (Groupe d'Etude des Lymphomes de l'Adulte) criteria.2 This is because MALT lymphoma frequently shows low 2-deoxy-2-[18F]fluoro-d-glucose uptake on PET,4 particularly in the stomach.5
Overexpression of the G protein–coupled C-X-C chemokine receptor type 4 (CXCR4) has been reported in MALT lymphoma.6 The aim of our exploratory study was therefore to evaluate PET with the novel CXCR4-targeting radiotracer [68Ga]Pentixafor as a potential noninvasive alternative to biopsies for assessment of residual MALT lymphoma after first-line H pylori eradication. [68Ga]Pentixafor–PET has performed well for staging of different blood cancers (including mantle cell, lymphoplasmacytic, and MALT lymphoma)7-15 and has shown promise for treatment response assessment.9,16
Study design
Patients and imaging
Patients with gastric MALT lymphoma, referred for follow-up esophagogastroduodenoscopy after H pylori eradication according to ESMO (European Society for Medical Oncology) guidelines,2 were prospectively included and underwent time-matched [68Ga]Pentixafor–PET/magnetic resonance imaging (MRI; within 28 days of endoscopy) as part of a larger exploratory study that investigated the feasibility of CXCR4 imaging in MALT lymphoma. The study was approved by the Ethics Committee of the Medical University of Vienna; written, informed consent was obtained from all patients. Histological verification of MALT lymphoma based on pathology specimens obtained from in-house or outside biopsies was performed by a reference hematopathologist according to World Health Organization criteria of lymphoid neoplasms.17 Pregnancy, breastfeeding, and contraindications to MRI (according to safety guidelines) were used as exclusion criteria. Twenty patients without gastric malignancies, who had undergone [68Ga]Pentixafor–PET/MRI for nonhematologic diseases, served as control group.
[68Ga]Pentixafor was synthesized as previously described.14 Whole-body [68Ga]Pentixafor–PET was performed on a hybrid PET/MRI device (Siemens Biograph mMR, Erlangen, Germany) 60 minutes after IV injection of 150 MBq of [68Ga]Pentixafor, with 5 minutes per bed position, 4 iterations, 21 subsets, a 4.2-mm slice thickness, and a 172 × 172 matrix. MRI was performed using a previously described protocol including axial T1-weighted and diffusion-weighted, and coronal T2-weighted sequences.14
Image analysis
[68Ga]Pentixafor-PET/MRIs were analyzed in random order by a board-certified radiologist and board-certified nuclear medicine physician, blinded to histology and prior imaging, in consensus. To avoid group bias, only PET images covering the anatomy from the aortic arch to the lowest point of the stomach were reviewed. The assessment was performed blinded to MRI; only when a positive finding could not be clearly attributed to the stomach on PET, then raters were given access to coregistered unenhanced T1-weighted sequences for anatomic correlation. PET was rated as positive (ie, noncomplete remission [non-CR]/residual disease in the MALT group) when unifocal or multifocal tracer accumulation was clearly discernible from the uptake of the surrounding/adjacent gastric wall/mucosa. On PET scans rated as non-CR, maximum and mean [68Ga]Pentixafor–PET standardized uptake values (SUVmax, SUVmean) were measured for gastric lesions, based on isocontour volumes of interest (VOIs) generated using the previously described 2.5-SUVmax cutoff.12 For comparative SUVs in the liver and blood pool (aortic arch), 1-cm3 spherical VOIs were used. After unblinding, whole-stomach SUVmax and SUVmean (based on isocontour VOIs) and liver and blood pool SUVs were also measured in the 20 controls and MALT lymphomas with false negative PET.
Reference standard
Biopsies obtained during esophagogastroduodenoscopy served as reference standard for all MALT lymphoma patients at each follow-up time point and were analyzed by 2 pathologists according to GELA response criteria.18,19 For control group patients, who did not undergo gastric biopsies, negative results on diagnostic MRI and negative follow-up imaging were used to rule out incidental gastric malignancies.
In all cases of biopsy-proven gastric lymphoma with sufficient tissue available, immunohistochemistry (IHC) with an antibody against CXCR4 (Epitomics, Burlingame, CA) was performed on 4-μm paraffin sections with a LEICA Bond III fully automated staining system, using the Bond Polymer Refine detection system and reagents supplied by Leica Microsystems (Newcastle-Upon-Tyne, United Kingdom), as previously described.20 Percentages of CXCR4+ lymphoma cells (showing membrane and/or cytoplasmic stainings, or dotlike cytoplasmic reaction) were estimated by 2 board-certified reference hematopathologists. In selected cases, double stainings were used to rule out CXCR4 expression by residual physiologic germinal center cells closely intermingled with lymphoma cells, using antibodies against BCL-6 or dendritic cells with antibodies against CD21. Images of hematoxylin-eosin (H&E)-stained tissue sections and IHC stains were taken with 10×/0.40 and 20×/0.80 objectives (Olympus UPlanXApo) of an Olympus BX53 microscope equipped with a ProgRes Gryphax NAOS digital camera, using the Jenoptik Gryphax software.
Statistical analysis
Pooled accuracy, sensitivity, specificity, and positive and negative predictive values of [68Ga]Pentixafor–PET (MALT lymphoma + control groups) were calculated per examination. General estimation equations that take multiple measurements per patient into account were used to calculate 95% confidence intervals (CIs). [68Ga]Pentixafor–PET tumor-to-background ratios were calculated as the quotient of lesion SUVmax and liver and blood pool SUVmax (TBRblood-max, TBRliver-max) or SUVmean (TBRblood-mean, TBRliver-mean). Stomach-to-background ratios (SBR) using gastric SUVmax measurements were also calculated. Correlation coefficients based on linear mixed models were used to determine associations between IHC-based percentages of CXCR4+ lymphoma cells and MALT lymphoma [68Ga]Pentixafor–PET metrics. The specified level of significance was P < .05. Analyses were performed using SPSS 24.0 (SPSS Inc, Chicago, IL).
Results and discussion
Forty-six post–H pylori eradication [68Ga]Pentixafor–PET/MRI examinations of 26 gastric MALT lymphoma patients (12 women and 14 men; mean age, 64.1 ± 11.9 years; range, 40-80 years) were performed in parallel to routine follow-up esophagogastroduodenoscopies (timing according to ESMO guidelines2). None of the patients showed an excess number of blasts or signs of transformation; all showed excellent performance status (ECOG 0-1). For histological features and treatments, see supplemental Table 1, available on the Blood Web site: 1 follow-up [68Ga]Pentixafor–PET/MRI was available in 17 patients; 2 PET/MRIs were available in 4 patients; 3 PET/MRIs were available in 2 patients; 4 PET/MRIs were available in 2 patients; and 7 PET/MRIs were available in 1 patient.
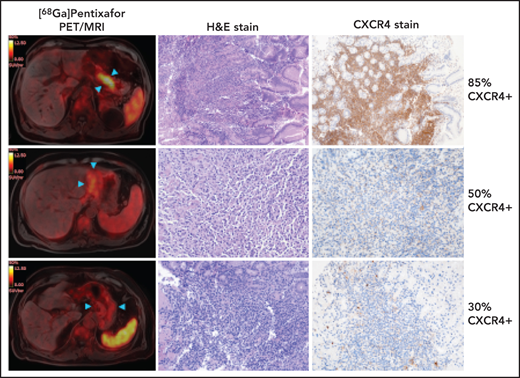
In the MALT lymphoma group, histology showed CR in 6 biopsies of 6 patients, and non-CR (residual disease) in the remaining 40 biopsies of 20 patients. In the 18 patients that were true positive on [68Ga]Pentixafor–PET, uptake was unifocal in 13 patients and multifocal in 5 patients. [68Ga]Pentixafor–PET was false negative in 2 of 40 cases (1 with diffuse, mild, pangastric uptake assumed to represent gastritis, as shown in Figure 1; the other without notable uptake), both of which showed low-level CXCR4 expression at time-matched biopsies/IHC (10% and 30% CXCR4+ lymphoma cells); and false positive in none (Figure 1). There was also no false positive [68Ga]Pentixafor–PET in the control group (12 patients with head/neck cancer; 5 patients with pancreatic ductal adenocarcinoma; 2 patients with vascular disease; and 1 patient with colorectal cancer). Accordingly, pooled examination-based accuracy, sensitivity, and specificity of [68Ga]Pentixafor–PET were 97.0% (CI, 88.4% to 99.3%), 95.0% (CI, 80.9% to 98.8%), and 100.0% (CI, 87.1%-100.0%), and positive and negative predictive values were 100.0% (CI, 90.8% to 100.0%) and 92.9% (CI, 75.5% to 98.2%), respectively. MALT lymphomas demonstrated high tumor-to-background contrast on [68Ga]Pentixafor–PET (Table 1); control group data suggest that the use of reference tissues, such as the liver, may possibly further improve [68Ga]Pentixafor–PET, especially in cases of diffuse involvement that may otherwise be misinterpreted (patient 22 in Figure 1).
Follow-up [68Ga]Pentixafor–PET/MRI after H pylori eradication in 4 gastric MALT lymphoma patients. (A) A 73-year-old man with marked focal gastric [68Ga]Pentixafor uptake (SUVmax, 10.8; blue arrowheads) rated as non-CR on PET/MRI. H&E stain confirms residual disease; roughly 85% of lymphoma cells show strong cytoplasmic and membranous reactivity with CXCR4 (original magnification ×100). (B) A 57-year-old man with masslike moderate gastric [68Ga]Pentixafor uptake (SUVmax, 6.4; blue arrowheads) rated as non-CR on PET/MRI. H&E stain confirms residual disease; ∼50% of lymphoma cells are weakly CXCR4+, showing dotlike paranuclear positivity (original magnification ×200). (C) A 73-year-old man with mild diffuse [68Ga]Pentixafor uptake (blue arrowheads), rated as CR on PET/MRI. However, H&E stain shows minor residual disease; ∼30% of lymphoma cells are weakly CXCR4+, again showing mostly paranuclear dotlike stainings (original magnification ×200). (D) A 49-year-old woman without increased [68Ga]Pentixafor uptake (arrowheads), rated as CR on PET/MRI. H&E stain confirms CR according to GELA criteria with focal complete intestinal metaplasia (original magnification ×100).
Follow-up [68Ga]Pentixafor–PET/MRI after H pylori eradication in 4 gastric MALT lymphoma patients. (A) A 73-year-old man with marked focal gastric [68Ga]Pentixafor uptake (SUVmax, 10.8; blue arrowheads) rated as non-CR on PET/MRI. H&E stain confirms residual disease; roughly 85% of lymphoma cells show strong cytoplasmic and membranous reactivity with CXCR4 (original magnification ×100). (B) A 57-year-old man with masslike moderate gastric [68Ga]Pentixafor uptake (SUVmax, 6.4; blue arrowheads) rated as non-CR on PET/MRI. H&E stain confirms residual disease; ∼50% of lymphoma cells are weakly CXCR4+, showing dotlike paranuclear positivity (original magnification ×200). (C) A 73-year-old man with mild diffuse [68Ga]Pentixafor uptake (blue arrowheads), rated as CR on PET/MRI. However, H&E stain shows minor residual disease; ∼30% of lymphoma cells are weakly CXCR4+, again showing mostly paranuclear dotlike stainings (original magnification ×200). (D) A 49-year-old woman without increased [68Ga]Pentixafor uptake (arrowheads), rated as CR on PET/MRI. H&E stain confirms CR according to GELA criteria with focal complete intestinal metaplasia (original magnification ×100).
[68Ga]Pentixafor–PET–based SUVs and TBR of biopsy-proven residual gastric MALT lymphomas after H pylori eradication; comparative control group [68Ga]Pentixafor–PET metrics
| . | Mean ± SE* . | Range* . | Correlation with IHC CXCR4+ cell %† . | P† . |
|---|---|---|---|---|
| MALT lymphoma | ||||
| SUVmax | 9.1 ± 0.74 | 3.3-18.2 | 0.51 | .008 |
| SUVmean | 4.5 ± 0.26 | 2.5-7.8 | 0.52 | .006 |
| TBRblood-max | 3.6 ± 0.28 | 1.3-7.3 | 0.42 | .014 |
| TBRliver-max | 4.2 ± 0.33 | 1.6-8.0 | 0.38 | .029 |
| TBRblood-mean | 5.1 ± 0.42 | 1.8-11.2 | 0.55 | .010 |
| TBRliver-mean | 7.4 ± 0.85 | 2.4-13.8 | 0.41 | .044 |
| Controls | ||||
| Gastric SUVmax | 3.2 ± 0.13 | 2.1-4.1 | NA | NA |
| Gastric SUVmean | 1.0 ± 0.10 | 1.2-2.7 | NA | NA |
| SBRblood-max | 1.1 ± 0.06 | 0.6-1.6 | NA | NA |
| SBRliver-max | 1.3 ± 0.06 | 0.8-1.7 | NA | NA |
| SBRblood-mean | 1.8 ± 0.11 | 0.9-2.9 | NA | NA |
| SBRliver-mean | 2.3 ± 0.14 | 1.3-3.3 | NA | NA |
| . | Mean ± SE* . | Range* . | Correlation with IHC CXCR4+ cell %† . | P† . |
|---|---|---|---|---|
| MALT lymphoma | ||||
| SUVmax | 9.1 ± 0.74 | 3.3-18.2 | 0.51 | .008 |
| SUVmean | 4.5 ± 0.26 | 2.5-7.8 | 0.52 | .006 |
| TBRblood-max | 3.6 ± 0.28 | 1.3-7.3 | 0.42 | .014 |
| TBRliver-max | 4.2 ± 0.33 | 1.6-8.0 | 0.38 | .029 |
| TBRblood-mean | 5.1 ± 0.42 | 1.8-11.2 | 0.55 | .010 |
| TBRliver-mean | 7.4 ± 0.85 | 2.4-13.8 | 0.41 | .044 |
| Controls | ||||
| Gastric SUVmax | 3.2 ± 0.13 | 2.1-4.1 | NA | NA |
| Gastric SUVmean | 1.0 ± 0.10 | 1.2-2.7 | NA | NA |
| SBRblood-max | 1.1 ± 0.06 | 0.6-1.6 | NA | NA |
| SBRliver-max | 1.3 ± 0.06 | 0.8-1.7 | NA | NA |
| SBRblood-mean | 1.8 ± 0.11 | 0.9-2.9 | NA | NA |
| SBRliver-mean | 2.3 ± 0.14 | 1.3-3.3 | NA | NA |
SE, standard error; NA, not applicable.
MALT group: based on 40 scans of 20 patients.
Based on 33 scans of 19 patients.
Thirty-three lymphoma specimens (time-matched to respective [68Ga]Pentixafor–PET/MRIs) of 19 of 20 patients with residual disease were available for IHC. Gastric MALT lymphomas were CXCR4+ in all cases, with a mean percentage of CXCR4+ cells of 58.5% ± 4.6% (range, 10% to 100%). Correlation between percentages of CXCR4+ cells and [68Ga]Pentixafor–PET metrics was moderate overall (Table 1), similar to previous studies in myeloma and mantle cell lymphoma.7,14 This is possibly because IHC is based on biopsy samples, whereas [68Ga]Pentixafor–PET SUVs are calculated for the entire lesion volume, thereby not reflecting local variations in CXCR4 expression.
Our results therefore suggest that CXCR4 PET imaging may possibly offer a reasonably sensitive and specific alternative to routine esophagogastroduodenoscopy for assessment of residual gastric MALT lymphoma (ie, CR vs non-CR) after H pylori eradication. We hypothesize that, in patients with markedly or moderately CXCR4+ disease according to IHC, a positive [68Ga]Pentixafor–PET may possibly obviate biopsies at each follow-up (ie, every 3 to 6 months in the case of asymptomatic residual disease2). In this scenario, biopsies could, theoretically, be used interchangeably with PET, unless transformation to large-cell lymphoma is suspected, for which [68Ga]Pentixafor–PET has not been tested. Additional studies are, however, clearly necessary to confirm observations in our small-sized cohort before any change in clinical management can be discussed.
Acknowledgments
The authors thank Friedrich Girschele for radiotracer synthesis or quality control, and Svenja Strohmer for her assistance in CXCR4 staining.
This work was funded, in part, through National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA008748 (M.E.M.).
Authorship
Contribution: M.E.M., M.R., and A.H. designed the research study; M.R., W.L., B.K., and A.L. recruited the patients; M.E.M., M.H., and I.S-K. provided financial resources and infrastructure for the study; L.N., S.S., and H.-J.W. were responsible for tracer development and production; M.E.M., A.H., I.S-K., and J.R. performed the image analysis; M.E.M. and M.W. performed the statistical analysis or data interpretation; M.E.M., M.R., A.H., I.S-K., B.K., and M.W. contributed to drafting/writing of the paper; and all authors critically revised the paper and approved the final version.
Conflict-of-interest disclosure: M.E.M. received speaker honoraria from Siemens, General Electric, and Bristol Myers Squibb. M.R. received speaker honoraria from Celgene, Ipsen, Novartis, and Eisai. H.-J.W. is a founder and shareholder of Scintomics. The remaining authors declare no competing financial interests.
Correspondence: Markus Raderer, Division of Oncology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; e-mail: markus.raderer@meduniwien.ac.at.
For original data, please contact mayerhom@mskcc.org.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Follow-up [68Ga]Pentixafor–PET/MRI after H pylori eradication in 4 gastric MALT lymphoma patients. (A) A 73-year-old man with marked focal gastric [68Ga]Pentixafor uptake (SUVmax, 10.8; blue arrowheads) rated as non-CR on PET/MRI. H&E stain confirms residual disease; roughly 85% of lymphoma cells show strong cytoplasmic and membranous reactivity with CXCR4 (original magnification ×100). (B) A 57-year-old man with masslike moderate gastric [68Ga]Pentixafor uptake (SUVmax, 6.4; blue arrowheads) rated as non-CR on PET/MRI. H&E stain confirms residual disease; ∼50% of lymphoma cells are weakly CXCR4+, showing dotlike paranuclear positivity (original magnification ×200). (C) A 73-year-old man with mild diffuse [68Ga]Pentixafor uptake (blue arrowheads), rated as CR on PET/MRI. However, H&E stain shows minor residual disease; ∼30% of lymphoma cells are weakly CXCR4+, again showing mostly paranuclear dotlike stainings (original magnification ×200). (D) A 49-year-old woman without increased [68Ga]Pentixafor uptake (arrowheads), rated as CR on PET/MRI. H&E stain confirms CR according to GELA criteria with focal complete intestinal metaplasia (original magnification ×100).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/2/10.1182_blood.2021013239/3/m_bloodbld2021013239f1.png?Expires=1768827887&Signature=qTxjnMeXX1ozWx5cST1wG-jZmcF0JGgELP2LwghOp7XPU76FNGg~vIvbhckSisv29KADJ8ikUC6MNpvRpp8~3bN7t6y58wgQD6OwuZtNG-cTt5GWKG3llLbL2SrVu4t9HdJuvVBIX7GpEYY-aj8zHjq2sDGboilpzkZLYrLZKMNrC70SNEfuEUogjYqwTwlthQt81vlboYIwfdP~qHxpWCbYlgz26I9tLvL7gbXrqJr7aKCvbvqXGr0GJ9iyemyBBjlCV05oM9FXZ6tt6eT62Pdxbj-0iMdKM3yDVKXumpfzCenv5U8SuFEQLZktdG6UVAD-1UfX0em6sJi~yn5m2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal