TO THE EDITOR:
Extracellular adenosine (eAdo) has been identified as a potent inhibitor of antitumor immune responses and enhancer of tumor survival. Two ectonucleotidases, CD39 and CD73, are involved in the generation of eAdo from adenosine triphosphate (ATP). Numerous studies have demonstrated their active role in promoting solid tumor outgrowth and spreading but also in inhibiting the antitumor immune response. Indeed, accumulation of eAdo was detected in solid tumors, and binding of eAdo to its receptors (A2AR) expressed at the surface of immune cells provides an immunosuppressive signal on effector T, natural killer (NK), and NKT cells, macrophages/dendritic cells, and neutrophils.1 In addition, signaling through A2AR upregulates a number of anti-inflammatory molecules and the activity of regulatory T cells, leading to a long-lasting immunosuppressive environment.2,3 Accordingly, the use of A2AR antagonists has demonstrated promising efficacy in the treatment of cancer.4 Anti-CD39 or anti-CD73 antibodies were also developed that efficiently block the hydrolysis of ATP into immune-suppressive eAdo and unleash antitumor immunity by stimulating dendritic cells and macrophages and restoring T-cell activation.5,6
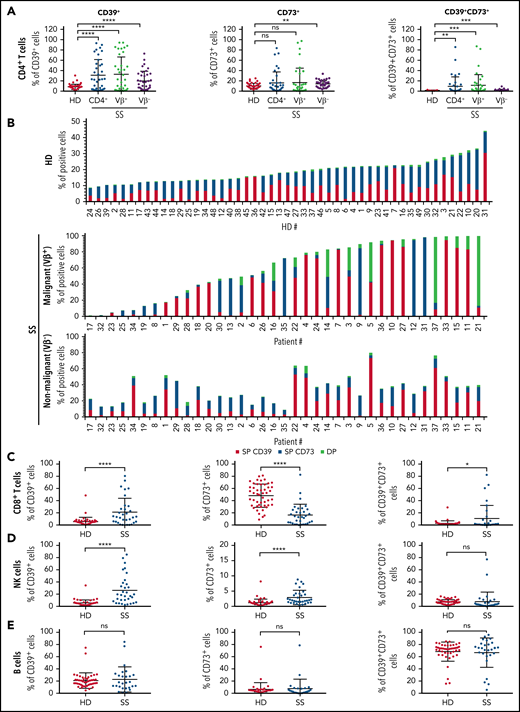
Sézary syndrome (SS) is an aggressive form of cutaneous T-cell lymphoma characterized by the presence of a malignant CD4+ T-cell clone in skin and blood and a global immunodeficiency.7 We previously demonstrated an abnormal expression of CD39 by the circulating tumor T cells in patients with SS,8 questioning the functional consequences of this overexpression in terms of antitumor immune response suppression and tumor cell growth. To gain insights into the potential role of the CD39/CD73/adenosine pathway in tumor escape, we further evaluated both CD39 and CD73 expression by circulating tumor CD4+ T-cell clone and nonmalignant T/B/NK lymphocytes in patients with SS. Multicolor flow cytometry analysis was performed on the blood of healthy donors (HD, n = 49) and patients with SS whose malignant CD4+ T-cell clone can be distinguished from the nonmalignant CD4+ T cells using an available anti-TCRVβ monoclonal antibody (mAb; n = 37). With this larger cohort, we confirmed the previously described overexpression of CD39 by Sézary patients' malignant CD4+ T-cell clone when compared with HD CD4+ T-cell population (Figure 1A; means ± standard deviation in supplemental Table 1 on the Blood Web site). Remarkably, addition of CD73 marker allowed us to distinguish 3 groups of patients according to CD39 and/or CD73 positivity of their tumor clone (Figure 1B): 51.3% (n = 19/37) and 27% (n = 10/37) of the patients had a tumor clone with a CD39+CD73− or CD39−CD73+ phenotype respectively, whereas 8.1% (n = 3/37) were characterized by a mainly CD39+CD73+ malignant clone (representative labelings shown in supplemental Figure 1A). No overexpression of either marker was detected on the malignant cells of 5/37 tested patients (13.5%). Significant overexpression of CD39 or CD73 by nonmalignant CD4+ T cells was also observed in SS compared with HD (Figure 1A-B; supplemental Table 1). However, it could be noted that, in a given patient, the nonmalignant CD4+ T cells and the malignant clone showed distinct CD39/CD73 expression profiles (Figure 1B), reinforcing the previously reported notion of genetic and phenotypic disease heterogeneity.9-12 Further comparison of the nonmalignant lymphocyte populations of SS patients vs HD revealed: (1) an up-modulation of CD39 and a down-modulation of CD73 on CD8+ T cells; (2) higher expression of CD39 or CD73 by NK cells; and (3) a similar CD39/CD73 phenotype on B cells (Figure 1C-E; supplemental Table 1). Altogether, these data highlight a strong bias in the expression profile of CD39 and/or CD73, not only on the tumor clone but also on the nonmalignant CD4+ and CD8+ T cells and NK cells, in the context of SS.
Abnormal expression of CD39 and CD73 on malignant and nonmalignant circulating lymphocytes of patients with SS. Expression of CD39 and CD73 was assessed on total blood of patients with SS (n = 37) or healthy donors (HD; n = 49) by flow cytometry. After acquisition on a flow cytometer, the percentage of CD39+ and/or CD73+ cells was estimated on (A) HD (CD3+CD4+) or SS total (CD3+CD4+), malignant (CD3+TCRVβ+CD4+) or nonmalignant (CD3+TCRVβ-CD4+) CD4+ T cells, (D) NK cells (CD3−CD56+) and (E) B cells (CD3−CD19+). (B) Histograms showing CD39 and CD73 single (SP) or double (DP) expression by normal (HD) or malignant/nonmalignant (SS) CD4+ T cells of each donor tested in each group. (A,C-E) Statistical analysis was performed using a Mann-Whitney t test. **P < .01, ***P < .001, ****P < .0001.
Abnormal expression of CD39 and CD73 on malignant and nonmalignant circulating lymphocytes of patients with SS. Expression of CD39 and CD73 was assessed on total blood of patients with SS (n = 37) or healthy donors (HD; n = 49) by flow cytometry. After acquisition on a flow cytometer, the percentage of CD39+ and/or CD73+ cells was estimated on (A) HD (CD3+CD4+) or SS total (CD3+CD4+), malignant (CD3+TCRVβ+CD4+) or nonmalignant (CD3+TCRVβ-CD4+) CD4+ T cells, (D) NK cells (CD3−CD56+) and (E) B cells (CD3−CD19+). (B) Histograms showing CD39 and CD73 single (SP) or double (DP) expression by normal (HD) or malignant/nonmalignant (SS) CD4+ T cells of each donor tested in each group. (A,C-E) Statistical analysis was performed using a Mann-Whitney t test. **P < .01, ***P < .001, ****P < .0001.
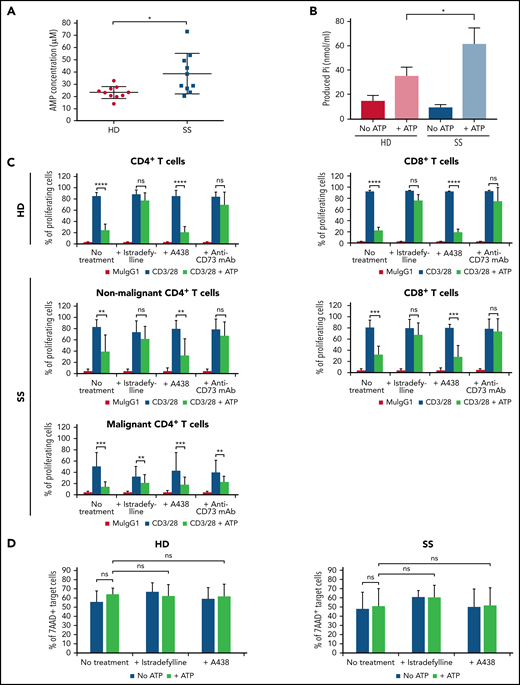
Despite high expression level of CD39 by the malignant cells of patients with SS, no significant increased release of soluble CD39 was detected in the plasma samples of patients with SS when compared with HD (supplemental Figure 1B). However higher levels of circulating adenosine 5′-monophosphate were observed (Figure 2A), as well as an increased ability of CD4+ T cells of patients with SS to promote ATP hydrolysis (Figure 2B). Because eAdo overproduction might occur from CD39/CD73 overexpression, we tested its possible impact on the T-cell functions of patients with SS. Proliferations assays were conducted on peripheral blood mononuclear cells (PBMC) activated with CD3/CD28 beads. As reported previously,13 the CD3/CD28-induced proliferation of HD CD4+ and CD8+ T cells was strongly inhibited in the presence of ATP (Figure 2C; n = 6). This ATP-induced inhibition was completely abolished when istradefylline, an antagonist inhibitor of the eAdo A2AR, or an anti-CD73 mAb previously identified as blocking CD73 nucleotidase activity,14 was added. In contrast A438079, an antagonist of the ATP receptors P2X7R, remained ineffective in preventing the effects of ATP (Figure 2C). Similar experiments performed on the PBMC of patients with SS (n = 6) revealed an identical CD8+ T-cell response as in HD, namely an ATP-mediated inhibition of proliferation that was abolished by addition of istradefylline or anti-CD73 mAb, but not A438079 (Figure 2C). Regarding CD4+ T cells, although malignant cells proliferated less than nonmalignant cells upon CD3/CD28 activation, both populations' proliferation was affected by ATP. However, abolition of the ATP-mediated inhibition of proliferation by istradefylline or the anti-CD73 mAb appeared fully effective on nonmalignant CD4+ T cells, but only partially on the malignant clone, which proliferation remained altered (Figure 2C). There again, A438079 did not prevent the inhibitory effects of ATP regardless of the CD4+ T-cell subtype. It therefore seemed that the ATP immunosuppressive function relied on the same mechanism in nonmalignant CD4+ and CD8+ T cells of patients with SS as in HD normal T cells, involving the conversion of ATP into eAdo by CD39/CD73 and eAdo binding to A2AR on T cells. In contrast, our data suggest that another mechanism prevailed in malignant CD4+ T cells as inhibition of eAdo production or eAdo/A2AR interaction was not sufficient by itself to fully prevent their ATP-induced inhibition of proliferation. As mentioned previously, malignant cells developed lower proliferation levels than their nonmalignant counterparts in response to CD3/CD28 activation (Figure 2A), a phenomenon previously attributed to antibody-induced cell death resistance.15 One could therefore hypothesize that part of the malignant cells are biased toward apoptosis resistance, remaining unresponsive to proliferative signals.
Impact of CD39/CD73 overexpression on malignant and nonmalignant T- and NK-cell functions. (A) Quantification of adenosine 5′-monophosphate in the plasma of HD and patients with SS (n = 10 per group). (B) Quantification of cell-associated CD39/CD73 enzymatic activity using purified CD4+ T cells from HD or patients with SS (n = 4 per group). (C) T-cell proliferation assay. Carboxyfluorescein diacetate succinimidyl ester- or CellTrace Violet-labeled PBMC from HD or patients with SS (n = 6 per group) were either left untreated or activated for 4 days with CD3/CD28 beads in the presence of ATP alone or combined with istradefylline (50 mM), A438079 (A438; 20 μM), or a blocking anti-CD73 mAb (10 μg/mL). Following immunolabeling, proliferation of the CD4+ and CD8+ T cells was assessed by flow cytometry. (D) ADCC assay. PBMC from HD or patients with SS were incubated with interleukin-15 in the presence of ATP alone or combined to istradefylline or A438. After 4 days, cells were mixed with Raji target cells at an E/T ratio of 20/1 and rituximab (10 μg/mL). Target cells apoptosis was monitored by 7-AAD labeling. Results are expressed as the mean ± standard deviation of the % of 7AAD+ Raji cells obtained for the indicated conditions with HD (left) and SS (right) PBMC (n = 4 per group). (A-D) Statistical analysis was performed using a Mann-Whitney t test. **P < .01, ***P < .001, ****P < .0001; ns, not significant.
Impact of CD39/CD73 overexpression on malignant and nonmalignant T- and NK-cell functions. (A) Quantification of adenosine 5′-monophosphate in the plasma of HD and patients with SS (n = 10 per group). (B) Quantification of cell-associated CD39/CD73 enzymatic activity using purified CD4+ T cells from HD or patients with SS (n = 4 per group). (C) T-cell proliferation assay. Carboxyfluorescein diacetate succinimidyl ester- or CellTrace Violet-labeled PBMC from HD or patients with SS (n = 6 per group) were either left untreated or activated for 4 days with CD3/CD28 beads in the presence of ATP alone or combined with istradefylline (50 mM), A438079 (A438; 20 μM), or a blocking anti-CD73 mAb (10 μg/mL). Following immunolabeling, proliferation of the CD4+ and CD8+ T cells was assessed by flow cytometry. (D) ADCC assay. PBMC from HD or patients with SS were incubated with interleukin-15 in the presence of ATP alone or combined to istradefylline or A438. After 4 days, cells were mixed with Raji target cells at an E/T ratio of 20/1 and rituximab (10 μg/mL). Target cells apoptosis was monitored by 7-AAD labeling. Results are expressed as the mean ± standard deviation of the % of 7AAD+ Raji cells obtained for the indicated conditions with HD (left) and SS (right) PBMC (n = 4 per group). (A-D) Statistical analysis was performed using a Mann-Whitney t test. **P < .01, ***P < .001, ****P < .0001; ns, not significant.
New therapeutic approaches in cutaneous T-cell lymphoma include depleting antibodies such as the recently developed anti-CCR4 (mogamulizumab)16 and anti-KIR3DL2 (IPH4102/lacutamab)17 antibodies. It has been demonstrated that NK cells actively participated in malignant cell depletion by exerting antibody-dependent cell cytotoxicity (ADCC).18,19 Inhibition of ADCC would therefore be detrimental for the obtention of maximal antitumor responses. We thus explored the impact of ATP/eAdo generation on NK cell ADCC function. Assays were conducted using interleukin-15-treated PBMC as source of activated NK cells, the CD20+ B-cell line Raji as target cells, and the anti-CD20 antibody rituximab as therapeutic antibody. Efficient killing of Raji cells was promoted by HD PBMC, which was not significantly affected by addition of ATP (Figure 2D, left). Consequently, addition of istradefylline or A438 did not interfere in the process leading to target cell depletion. Identical results were obtained with the PBMC of patients with SS (Figure 2D, right).
We here established that CD39 and/or CD73 cell surface expression is dysregulated on tumor clone of patients with SS but also on the nonmalignant T and NK cell populations, resulting in increased ATP hydrolysis. We further demonstrated that, in an ATP-enriched cellular environment, inhibition of the CD39/CD73/adenosine pathway could completely restore nonmalignant CD4+ and CD8+ T-cell proliferation but not malignant T-cell proliferation. Finally, the ADCC activity of NK cells did not seem affected by high ATP levels. In light of these data, the combined use of ADCC-driving therapeutic antibodies and antagonist inhibitors of CD39/CD73/adenosine pathway could represent an interesting option for improving antibody-dependent antitumor immune responses in the context of SS.
Acknowledgments
This work was supported by grants from INSERM, Paris Diderot University, and the French Society for Dermatology.
Authorship
Contribution: G.S., M.D., A. Bozonnat, and N.T. performed the experiments; A.M.-C. designed the research; A.M.-C., A. Bensussan, A.d.M., and M.B. analyzed the data; M.B., C.R.-W., and A.d.M. were attending doctors for the patients; and A.M.-C., M.B., A.d.M., and A. Bensussan wrote or critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Marie-Cardine, INSERM U976 Team 1, Hôpital Saint Louis, Pavillon Bazin, 1 avenue Claude Vellefaux, 75010 Paris, France; e-mail: anne.marie-cardine@inserm.fr.
The online version of this article contains a data supplement.
REFERENCES
Author notes
G.S., A. Bozonnat, and M.D. contributed equally to this study and are joint first authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal