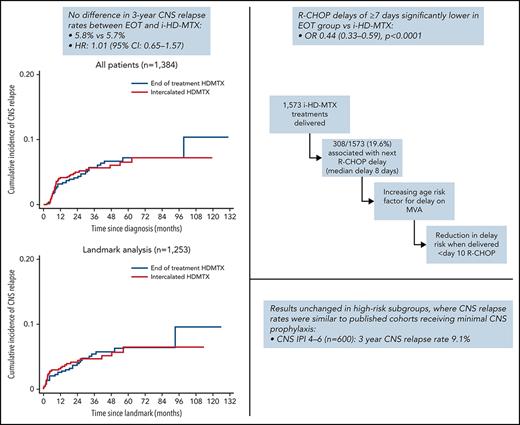
Abstract
Prophylactic high-dose methotrexate (HD-MTX) is often used for diffuse large B-cell lymphoma (DLBCL) patients at high risk of central nervous system (CNS) relapse, despite limited evidence demonstrating efficacy or the optimal delivery method. We conducted a retrospective, international analysis of 1384 patients receiving HD-MTX CNS prophylaxis either intercalated (i-HD-MTX) (n = 749) or at the end (n = 635) of R-CHOP/R-CHOP-like therapy (EOT). There were 78 CNS relapses (3-year rate 5.7%), with no difference between i-HD-MTX and EOT: 5.7% vs 5.8%, P = .98; 3-year difference: 0.04% (−2.0% to 3.1%). Conclusions were unchanged on adjusting for baseline prognostic factors or on 6-month landmark analysis (n = 1253). In patients with a high CNS international prognostic index (n = 600), the 3-year CNS relapse rate was 9.1%, with no difference between i-HD-MTX and EOT. On multivariable analysis, increasing age and renal/adrenal involvement were the only independent risk factors for CNS relapse. Concurrent intrathecal prophylaxis was not associated with a reduction in CNS relapse. R-CHOP delays of ≥7 days were significantly increased with i-HD-MTX vs EOT, with 308 of 1573 (19.6%) i-HD-MTX treatments resulting in a delay to subsequent R-CHOP (median 8 days). Increased risk of delay occurred in older patients when delivery was later than day 10 in the R-CHOP cycle. In summary, we found no evidence that EOT delivery increases CNS relapse risk vs i-HD-MTX. Findings in high-risk subgroups were unchanged. Rates of CNS relapse in this HD-MTX-treated cohort were similar to comparable cohorts receiving infrequent CNS prophylaxis. If HD-MTX is still considered for certain high-risk patients, delivery could be deferred until R-CHOP completion.
Key Points
End of treatment HD-MTX did not increase risk of CNS relapse compared with intercalated delivery and caused fewer delays to R-CHOP therapy.
CNS relapse rates in this large analysis of HD-MTX-treated patients were similar to published cohorts receiving minimal CNS prophylaxis.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL). Between 60% and 70% of cases are cured with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) immunochemotherapy.1 Systemic disease progression is the primary cause of treatment failure; however, relapse within the central nervous system (CNS) occurs in ∼2% to 5%2-4 with poor outcomes.5
The CNS international prognostic index (CNS-IPI) is the most established model for predicting CNS relapse risk and incorporates IPI factors plus an additional point for renal and/or adrenal involvement.6 Patients with CNS-IPI 4-6 have a risk of CNS relapse of ∼10%, and CNS-IPI ≥5 patients incur a risk of 15% to 30%. Although the CNS-IPI has improved on earlier models for selecting high-risk patients, the specificity remains unsatisfactory, subjecting many patients to unnecessary prophylaxis. Advances have been made in using molecular subtyping to identify patients at highest risk of CNS relapse, as well as using baseline cerebrospinal spinal fluid (CSF) circulating tumor DNA (ctDNA) assessment; however, this is costly, invasive, and these findings require validation in larger cohorts before being incorporated into routine practice.7,8
Various attempts have been made to incorporate CNS-penetrating prophylaxis into frontline therapy, aiming to minimize interruption of systemic treatment while reducing CNS relapses in those most at risk. There remains a lack of robust evidence to guide management, with national guidelines and position papers relying on mainly retrospective data to make pragmatic recommendations about prophylactic strategies.9 High-dose methotrexate (HD-MTX) is widely recommended as CNS prophylaxis in preference to intrathecal (IT) therapy as the majority of relapses are parenchymal, and the growing evidence suggests IT therapy alone is ineffective.10,11 Historical retrospective studies suggest that HD-MTX may be effective CNS prophylaxis,12-14 but no randomized trials have been performed to confirm this. Recent analyses cast doubt on HD-MTX efficacy, including a retrospective study of approximately 2300 patients demonstrating no apparent benefit in high-risk patients.15-19 Assuming HD-MTX may provide benefit to some high-risk patients, there is uncertainty over how to safely integrate this into frontline therapy. Advocates of an ‘intercalated' (i-HD-MTX) approach hypothesize that delivery between early cycles of R-CHOP may prevent very early CNS relapses, while others prefer delivering HD-MTX at the end of treatment (EOT) to avoid interruptions/delays to potentially curative systemic therapy.
We previously analyzed 334 patients treated with either i-HD-MTX or EOT HD-MTX.20 Delays to R-CHOP were significantly increased by i-HD-MTX compared with EOT, and although no differences in CNS relapse rate or survival between approaches were identified, the event rate was too low to draw definitive conclusions. Given the critical importance of maintaining dose intensity of systemic DLBCL therapy and the increasing scrutiny over HD-MTX efficacy as CNS prophylaxis, we conducted a large international study (n = 1384) with the primary aim of determining whether EOT HD-MTX is as effective as i-HD-MTX in preventing CNS relapse. Secondary endpoints included the impact of HD-MTX timing on survival, toxicity, and delays to R-CHOP cycles and risk factors for CNS relapse, including the influence of concurrent IT prophylaxis.
Methods
We conducted a multicenter retrospective analysis of patients ≥16 years with DLBCL or high-grade B-cell lymphoma not otherwise specified diagnosed between 2007 and 2020 from 47 centers in Europe, Australia, and North America. The study received ethical approval from the West of Scotland Research Ethics Committee (REC:20/WS/0114). Data were collected in compliance with national and/or local regulations and data transfer agreements used where required.
Patients were included if they received frontline R-CHOP or R-CHOP-like therapy with curative intent as well as HD-MTX CNS prophylaxis. HD-MTX was defined as any IV MTX dose intended to cross the blood-brain barrier and exert a prophylactic effect, given for ≥1 cycle. Diagnosis was established by local hematopathology review, with no central pathological review performed. Patients with previously untreated transformed low-grade NHL were included, and concurrent IT prophylaxis was permitted. Patients with HIV-associated DLBCL were included, but those with immunosuppression-related lymphoproliferative disorders and Burkitt lymphoma were excluded. Patients with known CNS involvement at diagnosis and those treated with more intensive regimens, including dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, rituximab (DA-EPOCH-R), were excluded. Baseline CNS evaluation was performed according to local clinician discretion.
Patient records were reviewed by local investigators. Data were recorded in a standardized, study-specific collection sheet and returned to principal investigators for secure central database storage.
Patients were selected for CNS prophylaxis according to local policies based on published risk models or due to the involvement of specific high-risk sites. Delivery of HD-MTX (i-HD-MTX or EOT) was determined according to local center preference, with i-HD-MTX defined as any patient receiving HD-MTX before the final R-CHOP cycle.
Standard baseline characteristics and prognostic indicators were recorded for all patients. Response to frontline therapy was recorded according to the Lugano classification.21 The number of delays to R-CHOP cycles of ≥7 days throughout therapy was recorded for all patients. All i-HD-MTX treatments were reviewed with the number of days delay to subsequent R-CHOP cycles reported.
We aimed to exclude a ≥5% difference in CNS relapse rate between EOT HD-MTX and i-HD-MTX (ie, that EOT HD-MTX was not more than 5% inferior), using a preplanned power calculation (supplementary Materials). Time-to-CNS relapse was calculated from diagnosis date until CNS relapse with systemic-only relapse and death in remission treated as competing events. Patients alive without relapse were censored at the date last seen. Analyses used competing risks by the Fine and Gray method. Time to isolated CNS relapse was analyzed in the same manner, but with concurrent systemic relapse (defined as CNS and systemic relapse occurring within 30 days of each other) also counting as a competing event. Due to violations in the proportional hazards (PHs) assumption for other prognostic factors of interest, an analysis using pseudo-observation methods22 (difference in 3-year cumulative incidence and lifetime lost over 10 years) was also performed. Progression-free survival (PFS) and overall survival (OS) were analyzed using Kaplan Meier survival analysis and Cox regression with times measured from the date of diagnosis until the first event, and patients without an event were censored at the date last seen. Treatment delays were analyzed using logistic regression (endpoint: any delay ≥7 days during chemotherapy) and mixed-effects logistic regression models (delays after each cycle of i-HD-MTX). Analyses were performed with STATA v16.1 (StataCorp, College Station, TX).
When identifying these patients in a retrospective manner, there is a risk that some patients planned for EOT HD-MTX are missed due to early progression. To address this potential survivorship bias in the EOT group, a secondary analysis for patients who had responded and were alive and progression-free at 6 months was also performed.
Results
Baseline characteristics for all 1384 patients (i-HD-MTX n = 749, EOT n = 635) are summarized in Table 1. Median follow-up was 37.9 months. Characteristics of i-HD-MTX and EOT groups were closely matched, with no statistically significant differences in risk factors included in the CNS-IPI except for advanced stage (i-HD-MTX 86.4% vs EOT 80.2%, P = .002). Overall, 44.2% had a CNS-IPI 4-6, 40.9% had a CNS-IPI 2-3, and 14.9% had a CNS-IPI 0-1. Applying the CNS relapse risk estimates from the validation cohort in the CNS-IPI publication (0.8%, 3.9%, and 12% for CNS-IPI risk groups, respectively), the estimated risk in our whole population was 7.0%. There was a trend toward a higher CNS-IPI score for i-HD-MTX patients (P = .083); however, there was no significant difference in the numbers with scores 4-6 (45.1% vs 43.0%, P = .45). The group with low CNS-IPI (n = 203) was enriched for patients considered to have a high-risk EN site involvement (181/203 [89.2%]), the most common of which were testicular (37.6%), craniofacial (22.1%), and breast (10.5%). Detailed reasons for CNS prophylaxis are in supplemental Table 1.
Baseline characteristics of the whole study population
| . | All . | End of treatment . | Intercalated . | P . |
|---|---|---|---|---|
| n = 1384 (%) . | n = 635 (%) . | n = 749 (%) . | ||
| Age (y), median (range) | 62.5 (17-88) | 63.0 (18-86) | 62.0 (17-88) | .065 |
| Follow-up (mo), median (IQR) | 37.9 (21.8-59.6) | 41.0 (25.0-63.2) | 35.2 (19.6-56.5) | |
| Baseline creatinine clearance, median (range) | 98.2 (33.3-345.2) | 94.5 (33.3-345.2) | 101.9 (35.5-332) | .0001 |
| Male sex | 840 (60.7) | 393 (61.9) | 447 (59.7) | .40 |
| Advanced stage | 1156 (83.5) | 509 (80.2) | 647 (86.4) | .0019 |
| Raised LDH baseline | 943 (70.0) | 410 (68.0) | 533 (71.5) | .16 |
| Missing/unknown | 36 | 32 | 4 | |
| ECOG ≥2 | 358 (25.9) | 158 (25.0) | 200 (26.7) | .47 |
| Missing/unknown | 3 | 3 | 0 | |
| Extranodal sites | ||||
| 0-1 | 586 (42.3) | 282 (44.4) | 304 (40.6) | .11* |
| 2 | 421 (30.4) | 191 (30.1) | 230 (30.7) | |
| ≥3 | 377 (27.2) | 162 (25.5) | 215 (28.7) | |
| Renal or adrenal involvement | 240 (17.3) | 102 (16.1) | 138 (18.4) | .25 |
| Testicular involvement | 175 (12.7) | 95 (15.0) | 80 (10.7) | .016 |
| Breast involvement | 56 (4.1) | 18 (2.8) | 38 (5.1) | .037 |
| Double or triple hit | 66 (6.1) | 32 (6.7) | 34 (5.7) | .47 |
| Missing/unknown | 308 | 159 | 149 | |
| CNS IPI | ||||
| Low (0-1) | 203 (14.9) | 107 (17.5) | 96 (12.9) | .083* |
| Intermediate (2-3) | 555 (40.9) | 241 (39.4) | 314 (42.0) | |
| High (4-6) | 600 (44.2) | 263 (43.0) | 337 (45.1) | |
| Missing/unknown | 26 | 24 | 2 | |
| Baseline CNS assessment | 703 (50.8) | 382 (60.2) | 321 (42.9) | <.0001 |
| . | All . | End of treatment . | Intercalated . | P . |
|---|---|---|---|---|
| n = 1384 (%) . | n = 635 (%) . | n = 749 (%) . | ||
| Age (y), median (range) | 62.5 (17-88) | 63.0 (18-86) | 62.0 (17-88) | .065 |
| Follow-up (mo), median (IQR) | 37.9 (21.8-59.6) | 41.0 (25.0-63.2) | 35.2 (19.6-56.5) | |
| Baseline creatinine clearance, median (range) | 98.2 (33.3-345.2) | 94.5 (33.3-345.2) | 101.9 (35.5-332) | .0001 |
| Male sex | 840 (60.7) | 393 (61.9) | 447 (59.7) | .40 |
| Advanced stage | 1156 (83.5) | 509 (80.2) | 647 (86.4) | .0019 |
| Raised LDH baseline | 943 (70.0) | 410 (68.0) | 533 (71.5) | .16 |
| Missing/unknown | 36 | 32 | 4 | |
| ECOG ≥2 | 358 (25.9) | 158 (25.0) | 200 (26.7) | .47 |
| Missing/unknown | 3 | 3 | 0 | |
| Extranodal sites | ||||
| 0-1 | 586 (42.3) | 282 (44.4) | 304 (40.6) | .11* |
| 2 | 421 (30.4) | 191 (30.1) | 230 (30.7) | |
| ≥3 | 377 (27.2) | 162 (25.5) | 215 (28.7) | |
| Renal or adrenal involvement | 240 (17.3) | 102 (16.1) | 138 (18.4) | .25 |
| Testicular involvement | 175 (12.7) | 95 (15.0) | 80 (10.7) | .016 |
| Breast involvement | 56 (4.1) | 18 (2.8) | 38 (5.1) | .037 |
| Double or triple hit | 66 (6.1) | 32 (6.7) | 34 (5.7) | .47 |
| Missing/unknown | 308 | 159 | 149 | |
| CNS IPI | ||||
| Low (0-1) | 203 (14.9) | 107 (17.5) | 96 (12.9) | .083* |
| Intermediate (2-3) | 555 (40.9) | 241 (39.4) | 314 (42.0) | |
| High (4-6) | 600 (44.2) | 263 (43.0) | 337 (45.1) | |
| Missing/unknown | 26 | 24 | 2 | |
| Baseline CNS assessment | 703 (50.8) | 382 (60.2) | 321 (42.9) | <.0001 |
P values are χ2 for discrete variables (*for trend) and Wilcoxon Mann-Whitney for continuous.
CNS IPI, central nervous system international prognostic index; ECOG, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; LDH, lactate dehydrogenase.
Patients with baseline positron emission tomography-computed tomography was 85%, and 50.8% had baseline CNS evaluation (9.3% CT or MRI and CSF analysis, 8.1% CT or MRI only, 33.4% CSF analysis only).
Treatment details, including HD-MTX delivery, are outlined in supplemental Table 2. Frontline immunochemotherapy was R-CHOP-21 (87.4%), R-CHOP-14 (9.4%), or R-CHOP-like therapy (3.2%); 91.8% received ≥6 cycles. Overall, 46.1% received IT prophylaxis in addition to HD-MTX, with significantly more in the EOT group compared with i-HD-MTX (55.7% vs 38.0%, P < .0001).
The median number of HD-MTX cycles delivered was 2 for both groups. Similar numbers received ≥2 cycles (87.7% vs 85.6%, P = .25); however, significantly more patients received ≥3 in the i-HD-MTX group (36.8% vs 12%, P < .0001) and the patient number receiving a total cumulative dose of >6 g/m2 HD-MTX was greater in the i-HD-MTX group (46.4% vs 23.2%, P < .0001).
There were 78 CNS relapses in the entire population (i-HD-MTX n = 41, EOT n = 37). CNS relapse was parenchymal in 41 (53%), parenchymal and leptomeningeal in 16 (21%), and leptomeningeal in 21 (27%) with similar distribution in both groups. The median time to CNS relapse was 8.5 months (interquartile range [IQR], 6.1-16.7) for the i-HD-MTX group and 10.3 months (IQR, 6.4-27.0) for the EOT group.
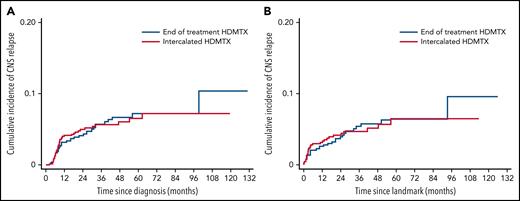
There was no difference in the 3-year CNS relapse rates between i-HDMTX and EOT groups: 5.7% vs 5.8%; hazard ratio (HR), 1.01; 95% CI, 0.65-1.57; P = .98 (Figure 1A). This remained similar when adjusted for baseline prognostic factors: HR, 1.06 (0.67-1.66); P = .82, and the 3-year difference (EOT - i-HD-MTX) excluded the noninferiority limit of +5% when calculated using the unadjusted or adjusted HR, difference: 0.04% (−2.0% to 3.1%) or 0.3% (−1.8% to 3.6%) (Table 2). On landmark analysis of patients alive and free from progression at 6 months (n = 1253), conclusions were unchanged: 3-year rates: 4.7% vs 4.7%, and 3-year differences of −0.03% (−1.0% to 3.0%) and −0.2% (−2.1% to 3.0%) using the unadjusted and adjusted HRs (Figure 1B). Baseline characteristics and details of events in excluded patients are described in supplemental Tables 3 and 4. Analyses performed using pseudo-observation methods also concurred.
Cumulative incidence of CNS relapse. (A) CNS relapse in the whole population, (B) CNS relapse in landmark population.
Cumulative incidence of CNS relapse. (A) CNS relapse in the whole population, (B) CNS relapse in landmark population.
Univariable and multivariable models for the difference in 3-y CNS relapse rates between i-HD-MTX and EOT groups, for all CNS relapses and for isolated CNS relapse only
| . | HR* (95% CI) . | 3-y difference, % (HR)† . | 3-y difference, %‡ . |
|---|---|---|---|
| All patients | |||
| EOT HD-MTX (UVA) | 1.01 (0.65-1.57) | 0.04 (−2.0 to 3.1) | 0.06 (−2.63 to 2.76) |
| EOT HD-MTX (adjusted§) | 1.06 (0.67-1.66) | 0.3 (−1.8 to 3.6) | 0.79 (−1.95 to 3.52) |
| EOT HD-MTX (adjustedǁ) | 0.07 (−2.59 to 2.73) | ||
| Landmark cohort only | |||
| EOT HD-MTX (UVA) | 0.99 (0.60-1.66) | −0.03 (−1.0 to 3.0%) | 0.02 (−2.58 to 2.63) |
| EOT HD-MTX (adjusted§) | 0.96 (0.55-1.67) | −0.2 (−2.1 to 3.0%) | 0.47 (−2.18 to 3.12) |
| EOT HD-MTX (adjustedǁ) | −0.11 (−2.70 to 2.48) | ||
| Isolated CNS relapse | |||
| EOT HD-MTX (UVA) | 1.07 (0.63-1.81) | 0.3 (−1.4 to 3.0%) | 0.47 (−1.84 to 2.78) |
| EOT HD-MTX (adjusted§) | 1.10 (0.64-1.87) | 0.4 (−1.4 to 3.2) | 1.00 (−1.38 to 3.30) |
| EOT HD-MTX (adjustedǁ) | 0.33 (−2.00 to 2.63) | ||
| Isolated CNS relapse, landmark cohort | |||
| EOT HD-MTX (UVA) | 1.07 (0.60-1.93) | 0.2 (−1.3 to 2.9%) | 1.11 (−1.34 to 3.56) |
| EOT HD-MTX (adjusted§) | 1.05 (0.57-1.95) | 0.2 (−1.7 to 3.6) | 1.02 (−1.33 to 3.37) |
| EOT HD-MTX (adjustedǁ) | 0.93 (−1.51 to 3.36) |
| . | HR* (95% CI) . | 3-y difference, % (HR)† . | 3-y difference, %‡ . |
|---|---|---|---|
| All patients | |||
| EOT HD-MTX (UVA) | 1.01 (0.65-1.57) | 0.04 (−2.0 to 3.1) | 0.06 (−2.63 to 2.76) |
| EOT HD-MTX (adjusted§) | 1.06 (0.67-1.66) | 0.3 (−1.8 to 3.6) | 0.79 (−1.95 to 3.52) |
| EOT HD-MTX (adjustedǁ) | 0.07 (−2.59 to 2.73) | ||
| Landmark cohort only | |||
| EOT HD-MTX (UVA) | 0.99 (0.60-1.66) | −0.03 (−1.0 to 3.0%) | 0.02 (−2.58 to 2.63) |
| EOT HD-MTX (adjusted§) | 0.96 (0.55-1.67) | −0.2 (−2.1 to 3.0%) | 0.47 (−2.18 to 3.12) |
| EOT HD-MTX (adjustedǁ) | −0.11 (−2.70 to 2.48) | ||
| Isolated CNS relapse | |||
| EOT HD-MTX (UVA) | 1.07 (0.63-1.81) | 0.3 (−1.4 to 3.0%) | 0.47 (−1.84 to 2.78) |
| EOT HD-MTX (adjusted§) | 1.10 (0.64-1.87) | 0.4 (−1.4 to 3.2) | 1.00 (−1.38 to 3.30) |
| EOT HD-MTX (adjustedǁ) | 0.33 (−2.00 to 2.63) | ||
| Isolated CNS relapse, landmark cohort | |||
| EOT HD-MTX (UVA) | 1.07 (0.60-1.93) | 0.2 (−1.3 to 2.9%) | 1.11 (−1.34 to 3.56) |
| EOT HD-MTX (adjusted§) | 1.05 (0.57-1.95) | 0.2 (−1.7 to 3.6) | 1.02 (−1.33 to 3.37) |
| EOT HD-MTX (adjustedǁ) | 0.93 (−1.51 to 3.36) |
The 10-y cut off for lifetime lost was chosen as close to the end of follow-up (131 mo, and after the last event).
CNS, central nervous system; ECOG, Eastern Cooperative Group performance status; EOT, end of treatment; HD-MTX, high-dose methotrexate; HR, hazard ratio; i-HD-MTX, intercalated high-dose methotrexate; IT, intrathecal; LDH, lactate dehydrogenase; UVA, univariate analysis.
HR for EOT vs i-HD-MTX.
Calculated by applying the hazard ratio to the 3-y rate in the i-HD-MTX group to get the corresponding rate in the EOT group, and then taking the difference.
Difference in cumulative incidence rates allowing for competing risks at 3 y using pseudo-observations.
Full model adjusted for sex, age, advanced stage, extra nodal disease (≥2 sites), ECOG (≥2), renal/adrenal involvement, raised LDH (plus ITs, HDMTX ≥2 doses, and cumulative dose >6 g/m2 for landmark cohort).
Adjusted for only variables significant with backward selection (based on survival time lost): age and renal/adrenal involvement for CNS relapse and age alone for isolated CNS relapse.
Subanalyses of CNS relapse in high-risk patients are summarized in Table 3. In patients with CNS-IPI 4-6 (n = 600) or CNS-IPI 5-6 (n = 210), the overall 3-year CNS relapse rates were 9.1% and 10.5%, respectively. Although this study was not powered for noninferiority comparisons within small high-risk subgroups, with the exception of breast involvement (n = 56 with only 5 events), all HRs were below or very close to 1, and 3-year differences between i-HD-MTX and EOT were under +0.2%. In a composite high-risk group (n = 885) including CNS-IPI 4-6 and/or any of the following: ≥3 extranodal sites, renal, adrenal, testicular, or breast involvement, there was no difference in 3-year CNS relapse rates between groups (i-HDMTX 7.4% vs EOT 7.7%; HR, 1.00; 95% CI, 0.61-1.62) and we could again exclude the +5% noninferiority margin; 3-year difference: 0.0% (−2.8 to 4.3). Applying the same subgroup analyses to the landmark cohort did not change these conclusions, and the 3-year difference within the composite high-risk group just met the noninferiority margin: 0.6% (−2.1% to 5.0%) (supplemental Table 5).
Results within specific high-risk groups
| . | 3-y CNS relapse rates, % . | Events/n . | HR* (95% CI) . | 3-y difference,% (EOT, intercalated) . |
|---|---|---|---|---|
| CNS IPI 4-6 | 9.1 (6.9-11.9) | 49/600 | ||
| Intercalated | 9.4 (6.5-13.5) | 28/337 | 1.00 | −0.7 (−4.4-5.4) |
| End of treatment | 8.6 (5.6-13.1) | 21/263 | 0.92 (0.52-1.62) | |
| CNS IPI 5-6 | 10.5 (5.9-16.0) | 21/210 | ||
| Intercalated | 11.8 (6.7-20.1) | 12/118 | 1.00 | −0.4 (−6.8-13.1) |
| End of treatment | 9.1 (4.6-17.4) | 9/92 | 0.96 (0.41-2.29) | |
| Testicular involvement | 7.5 (4.2-13.2) | 14/175 | ||
| Intercalated | 6.0 (2.3-15.3) | 8/80 | 1.00 | −0.4 (−4.0-9.3) |
| End of treatment | 8.5 (4.1-17.2) | 6/95 | 0.92 (0.32-2.68) | |
| Renal/adrenal involvement | 11.3 (7.6-16.7) | 25/240 | ||
| Intercalated | 14.4 (8.9-23.0) | 16/138 | 1.00 | −4.5 (−9.9-6.6) |
| End of treatment | 7.6 (3.7-15.5) | 9/102 | 0.67 (0.30-1.52) | |
| Breast involvement | 9.7 (3.6-24.6) | 5/56 | ||
| Intercalated | 5.3 (1.3-19.5) | 3/38 | 1.00 | 2.8 (−3.9-34.5) |
| End of treatment | 20.5 (5.6-60.3) | 2/18 | 1.56 (0.26-9.39) | |
| ≥3 extranodal sites | 7.6 (5.2-10.9) | 29/377 | ||
| Intercalated | 8.0 (5.0-12.8) | 16/215 | 1.00 | 0.0 (−4.1-8.1) |
| End of treatment | 7.1 (4.0-12.3) | 13/162 | 1.01 (0.48-2.10) | |
| Any high-risk factor above | 7.6 (5.9-9.7) | 65/885 | ||
| Intercalated | 7.4 (5.2-10.4) | 34/482 | 1.00 | 0.0 (−2.8-4.3) |
| End of treatment | 7.7 (5.3-11.1) | 31/403 | 1.00 (0.61-1.62) |
| . | 3-y CNS relapse rates, % . | Events/n . | HR* (95% CI) . | 3-y difference,% (EOT, intercalated) . |
|---|---|---|---|---|
| CNS IPI 4-6 | 9.1 (6.9-11.9) | 49/600 | ||
| Intercalated | 9.4 (6.5-13.5) | 28/337 | 1.00 | −0.7 (−4.4-5.4) |
| End of treatment | 8.6 (5.6-13.1) | 21/263 | 0.92 (0.52-1.62) | |
| CNS IPI 5-6 | 10.5 (5.9-16.0) | 21/210 | ||
| Intercalated | 11.8 (6.7-20.1) | 12/118 | 1.00 | −0.4 (−6.8-13.1) |
| End of treatment | 9.1 (4.6-17.4) | 9/92 | 0.96 (0.41-2.29) | |
| Testicular involvement | 7.5 (4.2-13.2) | 14/175 | ||
| Intercalated | 6.0 (2.3-15.3) | 8/80 | 1.00 | −0.4 (−4.0-9.3) |
| End of treatment | 8.5 (4.1-17.2) | 6/95 | 0.92 (0.32-2.68) | |
| Renal/adrenal involvement | 11.3 (7.6-16.7) | 25/240 | ||
| Intercalated | 14.4 (8.9-23.0) | 16/138 | 1.00 | −4.5 (−9.9-6.6) |
| End of treatment | 7.6 (3.7-15.5) | 9/102 | 0.67 (0.30-1.52) | |
| Breast involvement | 9.7 (3.6-24.6) | 5/56 | ||
| Intercalated | 5.3 (1.3-19.5) | 3/38 | 1.00 | 2.8 (−3.9-34.5) |
| End of treatment | 20.5 (5.6-60.3) | 2/18 | 1.56 (0.26-9.39) | |
| ≥3 extranodal sites | 7.6 (5.2-10.9) | 29/377 | ||
| Intercalated | 8.0 (5.0-12.8) | 16/215 | 1.00 | 0.0 (−4.1-8.1) |
| End of treatment | 7.1 (4.0-12.3) | 13/162 | 1.01 (0.48-2.10) | |
| Any high-risk factor above | 7.6 (5.9-9.7) | 65/885 | ||
| Intercalated | 7.4 (5.2-10.4) | 34/482 | 1.00 | 0.0 (−2.8-4.3) |
| End of treatment | 7.7 (5.3-11.1) | 31/403 | 1.00 (0.61-1.62) |
High risk CNS IPI: 9.5% (6.2-14.4) EOT and 9.4% (6.5-13.5) intercalated. High risk (all factors): 9.5% (6.6-13.5) EOT and 8.6% (5.9-12.4) intercalated.
CNS IPI, central nervous system international prognostic index; EOT, end of treatment; HR, hazard ratio.
EOT vs intercalated. Events post 3 years: 8 events (5 EOT and 3 intercalated). Five-year rates: EOT: 7.3% (5.2-10.1) and 6.5 (4.7-9.1) intercalated.
Univariable and multivariable analyses of risk factors for CNS relapse in the whole population and landmark cohort are described in Table 4. Multiple variables violated the PH assumption in both univariable and multivariable analysis, so an analysis was performed using a method comparing the expected CNS relapse-free “lifetime lost” over 10 years, allowing for systemic-only relapse and death in remission as competing events. Age and renal/adrenal involvement were the only independent risk factors in whole cohort and landmark analyses. Due to the potential for immortal time bias, other treatment parameters (use of concurrent IT prophylaxis, HD-MTX cycle number given, and cumulative HD-MTX dosage) were included only in landmark analyses. There was no evidence of associations with time to CNS relapse nor of interactions with HD-MTX timing.
Univariable and multivariable analyses of risk factors for all CNS relapse and isolated CNS relapse only
| Risk factor . | All patients . | Landmark . | ||
|---|---|---|---|---|
| Survival time lost (mo) . | P . | Survival time lost (mo) . | P . | |
| All CNS relapses, UVA | ||||
| EOT HD-MTX | 0.52 (−3.04-4.09) | .77 | 0.43 (−3.13-3.99) | .82 |
| Sex | 0.71 (−2.99-4.40) | .71 | 0.14 (−3.58-3.85) | .94 |
| Age (for a 10-y increase) | 1.61 (0.58-2.64) | .002 | 1.64 (0.61-2.66) | .002 |
| Advanced stage | 2.53 (−2.27-7.33) | .30 | 1.22 (−3.66-6.11) | .62 |
| Extranodal sites ≥2 | 4.39 (1.00-7.79) | .011 | 1.99 (−1.48-5.47) | .26 |
| ECOG ≥2 | 0.86 (−2.94-4.67) | .66 | 0.40 (−3.39-4.19) | .84 |
| Renal/adrenal involvement | 7.64 (2.28-13.00) | .005 | 6.06 (0.62-11.51) | .029 |
| Raised LDH | 3.02 (−0.29-6.34) | .074 | 1.63 (−1.67-4.94) | .33 |
| ITs given | 1.10 (−2.48-4.68) | .55 | ||
| HD-MTX doses ≥2 | −2.87 (−8.57-2.84) | .33 | ||
| Cumulative dose >6 g/m2 | −2.19 (−5.47-1.09) | .19 | ||
| All CNS relapses, MVA | ||||
| Age (for a 10-y increase) | 1.60 (0.59-2.61) | .002 | 1.33 (0.39-2.27) | .006 |
| Renal/adrenal involvement | 7.65 (2.31-13.00) | .005 | 5.45 (0.23-10.66) | .041 |
| Isolated CNS relapse, UVA | ||||
| EOT HD-MTX | 0.71 (−2.51-3.94) | .66 | 0.79 (−2.93-4.51) | .68 |
| Sex | 0.46 (−2.89-3.81) | .79 | 0.59 (−3.39-4.56) | .77 |
| Age (for a 10-y increase) | 1.42 (0.51-2.34) | .002 | 1.47 (0.44-2.49) | .005 |
| Advanced stage | 0.24 (−4.48-4.95) | .92 | −0.52 (−5.81-4.77) | .85 |
| Extranodal sites ≥2 | 2.21 (−0.89-5.31) | .16 | 0.82 (−2.79-4.42) | .66 |
| ECOG ≥ 2 | −0.69 (−3.90-2.52) | .67 | −1.63 (−5.11-1.85) | .36 |
| Renal/adrenal involvement | 3.89 (−0.54-8.32) | .086 | 2.29 (2.45-7.03) | .34 |
| Raised LDH | 1.17 (−1.86-4.19) | .45 | 0.03 (−3.27-3.32) | .99 |
| ITs given | 1.21 (−2.59-5.00) | .53 | ||
| HD-MTX doses ≥2 | −2.43 (−7.95-3.10) | .39 | ||
| Cumulative dose >6 g/m2 | −3.59 (−6.84 to −0.35) | .030 | ||
| Isolated CNS relapse, MVA | ||||
| Age (for a 10-y increase) | 1.41 (0.52-2.31) | .002 | 1.47 (−0.44-2.49) | .005 |
| Risk factor . | All patients . | Landmark . | ||
|---|---|---|---|---|
| Survival time lost (mo) . | P . | Survival time lost (mo) . | P . | |
| All CNS relapses, UVA | ||||
| EOT HD-MTX | 0.52 (−3.04-4.09) | .77 | 0.43 (−3.13-3.99) | .82 |
| Sex | 0.71 (−2.99-4.40) | .71 | 0.14 (−3.58-3.85) | .94 |
| Age (for a 10-y increase) | 1.61 (0.58-2.64) | .002 | 1.64 (0.61-2.66) | .002 |
| Advanced stage | 2.53 (−2.27-7.33) | .30 | 1.22 (−3.66-6.11) | .62 |
| Extranodal sites ≥2 | 4.39 (1.00-7.79) | .011 | 1.99 (−1.48-5.47) | .26 |
| ECOG ≥2 | 0.86 (−2.94-4.67) | .66 | 0.40 (−3.39-4.19) | .84 |
| Renal/adrenal involvement | 7.64 (2.28-13.00) | .005 | 6.06 (0.62-11.51) | .029 |
| Raised LDH | 3.02 (−0.29-6.34) | .074 | 1.63 (−1.67-4.94) | .33 |
| ITs given | 1.10 (−2.48-4.68) | .55 | ||
| HD-MTX doses ≥2 | −2.87 (−8.57-2.84) | .33 | ||
| Cumulative dose >6 g/m2 | −2.19 (−5.47-1.09) | .19 | ||
| All CNS relapses, MVA | ||||
| Age (for a 10-y increase) | 1.60 (0.59-2.61) | .002 | 1.33 (0.39-2.27) | .006 |
| Renal/adrenal involvement | 7.65 (2.31-13.00) | .005 | 5.45 (0.23-10.66) | .041 |
| Isolated CNS relapse, UVA | ||||
| EOT HD-MTX | 0.71 (−2.51-3.94) | .66 | 0.79 (−2.93-4.51) | .68 |
| Sex | 0.46 (−2.89-3.81) | .79 | 0.59 (−3.39-4.56) | .77 |
| Age (for a 10-y increase) | 1.42 (0.51-2.34) | .002 | 1.47 (0.44-2.49) | .005 |
| Advanced stage | 0.24 (−4.48-4.95) | .92 | −0.52 (−5.81-4.77) | .85 |
| Extranodal sites ≥2 | 2.21 (−0.89-5.31) | .16 | 0.82 (−2.79-4.42) | .66 |
| ECOG ≥ 2 | −0.69 (−3.90-2.52) | .67 | −1.63 (−5.11-1.85) | .36 |
| Renal/adrenal involvement | 3.89 (−0.54-8.32) | .086 | 2.29 (2.45-7.03) | .34 |
| Raised LDH | 1.17 (−1.86-4.19) | .45 | 0.03 (−3.27-3.32) | .99 |
| ITs given | 1.21 (−2.59-5.00) | .53 | ||
| HD-MTX doses ≥2 | −2.43 (−7.95-3.10) | .39 | ||
| Cumulative dose >6 g/m2 | −3.59 (−6.84 to −0.35) | .030 | ||
| Isolated CNS relapse, MVA | ||||
| Age (for a 10-y increase) | 1.41 (0.52-2.31) | .002 | 1.47 (−0.44-2.49) | .005 |
Survival time is measured up to 10 y; for example, in univariable analysis, a patient given EOT HDMTX has a CNS-relapse-free life expectancy over 10 y that is 0.43 mo shorter than for a patient given i-HD-MTX. The MVA shows variables remaining significant with backward selection (P value for rejection =.05). With a rare event, lifetime lost is not easily clinically interpretable, but at 3 years, this translates to a difference in cumulative incidence of 6.58% for patients with renal and adrenal involvement when compared with those without, and an increase in incidence of 1.12% for each decade of age.
ECOG, Eastern Cooperative Group performance status; EOT, end of treatment; HD-MTX, high-dose methotrexate; IT, intrathecal; LDH, lactate dehydrogenase; MVA, multivariable analysis; UVA, univariable analysis.
CNS relapses were isolated in 57 of 78 (73.1%) cases, with the remainder occurring in combination with systemic progression. Sites of isolated relapse were parenchymal in 35 of 57 (61%), leptomeningeal in 16 of 57 (28%), and both in 6 of 57 (11%). Median times to isolated CNS relapse in the i-HD-MTX and EOT groups were 8.3 months (IQR 6.1-18.2) and 12.2 (7.4-29.2) months, respectively. There was no difference in the 3-year cumulative incidence of isolated CNS relapse between groups (Table 4).
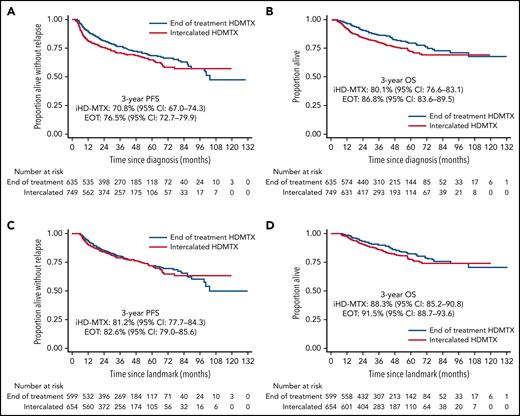
With a median follow-up of 37 months, PFS and OS were significantly inferior in the i-HD-MTX group compared with EOT, with differences persisting in a model adjusted for sex, age, ECOG performance status, presence of ≥2 EN sites, renal/adrenal involvement, and stratified by stage and lactate dehydrogenase (LDH) (PH violations): adjusted PFS HR, 0.79; 95% CI 0.64-0.98; P = .024; and OS HR, 0.67; 95% CI, 0.52-0.88; P = .003 (Figure 2A-B). However, on landmark analysis, there was no significant difference in PFS or OS between groups in univariable or adjusted analysis (model including aforementioned baseline characteristics as well as treatment parameters and chemotherapy delays): adjusted PFS HR, 1.05; 95% CI, 0.81-1.36; P = .72; and OS HR, 0.85; 95% CI, 0.61-1.18; P = .32 (Figure 2C-D).
Progression-free and overall survival. Whole cohort (A-B) and landmark cohort (C-D).
Progression-free and overall survival. Whole cohort (A-B) and landmark cohort (C-D).
Nonrelapse mortality (NRM) was reported in 55 of 1384 (4.0%) patients. Although no NRM events were reported as being directly attributable to HD-MTX, there was a trend toward a higher 3-year cumulative incidence of NRM in the i-HD-MTX group compared with EOT (3.9% vs 2.4%; HR, 0.60; 95% CI, 0.34-1.04; P = .06) (supplemental Figure 1). This did not seem to be driven by deaths during treatment as the landmark analysis remained similar: HR, 0.56; 95% CI, 0.31-1.02; P = .055.
The median OS of the 78 patients experiencing any CNS relapse was 5.4 months (IQR, 2.8-6.9), with no survival difference between i-HD-MTX and EOT groups (supplemental Figure 2A). When analyzed according to the presence of isolated CNS or synchronous systemic/CNS relapse, there was a trend toward inferior survival in patients with synchronous relapse (HR, 1.69; 95% CI, 0.96-2.98; P = .069) (supplemental Figure 2B). There was no difference in survival according to the site of CNS relapse (parenchymal vs leptomeningeal vs both) (supplemental Figure 2C).
Univariable and multivariable analyses of risk factors for any delay of ≥7 days during frontline therapy are displayed in Table 5. The only significant risk factor for delays was i-HD-MTX delivery (odds ratio, 0.44; 95% CI, 0.33-0.59; P < .0001). Results were unchanged using ordinal regression with the number of delays throughout therapy categorized as 0, 1 to 2, and ≥3.
Univariable and multivariable analyses of risk factors for any delay of ≥7 d during frontline therapy
| Risk factor . | Univariable . | Multivariable . | |||
|---|---|---|---|---|---|
| Events/n . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| ≥7-d delay (all patients) | |||||
| HD-MTX approach | |||||
| Intercalated | 196/743 | 1.00 | <.0001 | 1.00 | <.0001 |
| EOT | 79/616 | 0.41 (0.31-0.55) | 0.44 (0.33-0.59) | ||
| Age (for an increase of 10 y) | 275/1359 | 0.96 (0.87-1.06) | .37 | 0.92 (0.82-1.04) | .20 |
| Sex | |||||
| Male | 166/825 | 1.00 | .90 | 1.00 | .95 |
| Female | 109/534 | 1.02 (0.78-1.33) | 0.99 (0.75-1.32) | ||
| Advanced stage | |||||
| Stage 1-2 | 46/221 | 1.00 | .82 | 1.00 | .90 |
| Stage 3-4 | 229/1138 | 0.96 (0.67-1.37) | 0.97 (0.63-1.50) | ||
| ECOG | |||||
| 0-1 | 210/1004 | 1.00 | .32 | 1.00 | .43 |
| 2+ | 65/353 | 0.85 (0.63-1.16) | 0.88 (0.63-1.22) | ||
| 2+ extranodal sites | |||||
| <2 | 115/576 | 1.00 | .83 | 1.00 | .62 |
| 2+ | 160/783 | 1.03 (0.79-1.35) | 1.08 (0.79-1.48) | ||
| LDH | |||||
| Normal | 93/401 | 1.00 | .12 | 1.00 | .088 |
| >ULN | 180/925 | 0.80 (0.60-1.06) | 0.76 (0.56-1.04) | ||
| Baseline CrCl | 272/1321 | 0.94 (0.68-1.30) | .71 | 0.73 (0.49-1.10) | .14 |
| Risk factor . | Univariable . | Multivariable . | |||
|---|---|---|---|---|---|
| Events/n . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| ≥7-d delay (all patients) | |||||
| HD-MTX approach | |||||
| Intercalated | 196/743 | 1.00 | <.0001 | 1.00 | <.0001 |
| EOT | 79/616 | 0.41 (0.31-0.55) | 0.44 (0.33-0.59) | ||
| Age (for an increase of 10 y) | 275/1359 | 0.96 (0.87-1.06) | .37 | 0.92 (0.82-1.04) | .20 |
| Sex | |||||
| Male | 166/825 | 1.00 | .90 | 1.00 | .95 |
| Female | 109/534 | 1.02 (0.78-1.33) | 0.99 (0.75-1.32) | ||
| Advanced stage | |||||
| Stage 1-2 | 46/221 | 1.00 | .82 | 1.00 | .90 |
| Stage 3-4 | 229/1138 | 0.96 (0.67-1.37) | 0.97 (0.63-1.50) | ||
| ECOG | |||||
| 0-1 | 210/1004 | 1.00 | .32 | 1.00 | .43 |
| 2+ | 65/353 | 0.85 (0.63-1.16) | 0.88 (0.63-1.22) | ||
| 2+ extranodal sites | |||||
| <2 | 115/576 | 1.00 | .83 | 1.00 | .62 |
| 2+ | 160/783 | 1.03 (0.79-1.35) | 1.08 (0.79-1.48) | ||
| LDH | |||||
| Normal | 93/401 | 1.00 | .12 | 1.00 | .088 |
| >ULN | 180/925 | 0.80 (0.60-1.06) | 0.76 (0.56-1.04) | ||
| Baseline CrCl | 272/1321 | 0.94 (0.68-1.30) | .71 | 0.73 (0.49-1.10) | .14 |
A more conservative analysis which excluded any patient in the iHDMTX group given <6 cycles of treatment (ie, a patient group who may not have been given EOT MTX even if it was the intention) found very similar results for treatment approach: HR: 0.44 (0.33-0.59), P < .001 (UVA); and HR 0.47 (0.35-0.64), P < .001 (MVA).
CI, confidence interval; CrCl, creatinine clearance; ECOG, Eastern Cooperative Group performance status; EOT, intercalated; HD-MTX, high-dose methotrexate; LDH, lactate dehydrogenase; OR, odds ratio; ULN, upper limit of normal.
A total of 1573 cycles of HD-MTX were given intercalated between cycles of R-CHOP/R-CHOP-like therapy, with most patients receiving first HD-MTX delivery after cycle 1 or 2 (28.5% and 44.4%, respectively) (supplemental Figure 3A-B). The median day post-R-CHOP of i-HD-MTX delivery was 10 (IQR, 1-14), and the median number of intercalated cycles per patient was 2 (IQR, 1-2). Of the 1573 intercalated HD-MTX cycles, 308 (19.6%) resulted in subsequent R-CHOP delay (median delay 8 days [IQR, 6-19]).
Survival analyses in the landmark cohort demonstrated a significantly inferior PFS in patients who had a delay of ≥7 days vs those who did not (adjusted HR, 1.52; 95% CI, 1.15-2.03; P = .004) and a trend toward inferior OS (adjusted HR, 1.38; 95% CI, 0.96-1.98; P = .085).
Univariable and multivariable analyses of risk factors for delays following i-HD-MTX are displayed in Table 6. Increasing age and baseline creatinine clearance were the only significant factors associated with delays on univariate analysis, with increasing age the only variable approaching statistical significance on multivariate analysis (P = .055). Clinicians reported infection (19.5%), renal toxicity (11.7%), cytopenias (11.7%), administrative (8.1%), and mucositis (3.9%) as the most frequent reasons for delays after i-HD-MTX. Mixed-effects logistic regression models were used to assess delays at each cycle of i-HD-MTX (see supplemental Materials for full details). The only baseline factor significant in this analysis was older age, though there were interactions with dose and timing, which suggested that the increase in risk was only present for patients treated with higher doses (≥3 g/m2) and later in the R-CHOP cycle (>10 days). There was no clear evidence that delays were associated with the R-CHOP cycle in which the dose was given or the i-HD-MTX dose number.
Risk factors for delays following intercalated HD-MTX
| Risk factor . | Univariable . | Multivariable . | |||
|---|---|---|---|---|---|
| Events/n . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Age (for an increase of 10 y) | 214/748 | 1.20 (1.05-1.36) | .006 | 1.16 (1.00-1.35) | .055 |
| Sex | |||||
| Male | 131/447 | 1.00 | .61 | 1.00 | .74 |
| Female | 83/301 | 0.92 (0.66-1.27) | 0.95 (0.67-1.33) | ||
| Advanced stage | |||||
| Stage 1-2 | 30/102 | 1.00 | .85 | 1.00 | .82 |
| Stage 3-4 | 184/646 | 0.96 (0.60-1.51) | 1.06 (0.63-1.81) | ||
| ECOG | |||||
| 0-1 | 163/548 | 1.00 | .26 | 1.00 | .37 |
| 2+ | 51/200 | 0.81 (0.56-1.17) | 0.84 (0.57-1.23) | ||
| 2+ extranodal sites | |||||
| <2 | 87/303 | 1.00 | .96 | 1.00 | .98 |
| 2+ | 127/445 | 0.99 (0.72-1.37) | 1.00 (0.70-1.45) | ||
| LDH | |||||
| Normal | 69/212 | 1.00 | .15 | 1.00 | .21 |
| >ULN | 145/532 | 0.78 (0.55-1.10) | 0.79 (0.54-1.15) | ||
| Baseline CrCl (for an increase of 100) | 212/738 | 0.66 (0.44-0.99) | .043 | 0.84 (0.52-1.37) | .48 |
| Risk factor . | Univariable . | Multivariable . | |||
|---|---|---|---|---|---|
| Events/n . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Age (for an increase of 10 y) | 214/748 | 1.20 (1.05-1.36) | .006 | 1.16 (1.00-1.35) | .055 |
| Sex | |||||
| Male | 131/447 | 1.00 | .61 | 1.00 | .74 |
| Female | 83/301 | 0.92 (0.66-1.27) | 0.95 (0.67-1.33) | ||
| Advanced stage | |||||
| Stage 1-2 | 30/102 | 1.00 | .85 | 1.00 | .82 |
| Stage 3-4 | 184/646 | 0.96 (0.60-1.51) | 1.06 (0.63-1.81) | ||
| ECOG | |||||
| 0-1 | 163/548 | 1.00 | .26 | 1.00 | .37 |
| 2+ | 51/200 | 0.81 (0.56-1.17) | 0.84 (0.57-1.23) | ||
| 2+ extranodal sites | |||||
| <2 | 87/303 | 1.00 | .96 | 1.00 | .98 |
| 2+ | 127/445 | 0.99 (0.72-1.37) | 1.00 (0.70-1.45) | ||
| LDH | |||||
| Normal | 69/212 | 1.00 | .15 | 1.00 | .21 |
| >ULN | 145/532 | 0.78 (0.55-1.10) | 0.79 (0.54-1.15) | ||
| Baseline CrCl (for an increase of 100) | 212/738 | 0.66 (0.44-0.99) | .043 | 0.84 (0.52-1.37) | .48 |
MVA, with backward selection (P = .05 for inclusion), age is the only factor that remains: OR: 1.19 (1.05-1.35), P = .008 (n = 735). Note, this is slightly different from the UVA quoted (despite being the only variable left) as it included complete cases only.
See Table 5 for definitions.
The most frequent toxicities observed post-HD-MTX administration were febrile neutropenia, renal toxicity, and mucositis. No direct comparison between i-HD-MTX and EOT groups is possible, as some events for i-HD-MTX may be related to concurrent systemic chemotherapy. However, we observed numerically greater febrile neutropenia (15.2% vs 2.5%), mucositis (15.4% vs 4.6%), and renal toxicity (17.8% vs 13.9%) in patients in i-HD-MTX vs EOT.
Discussion
Most DLBCL patients are cured with frontline chemoimmunotherapy, and there have been significant advances in recent years for patients with relapsed/refractory systemic disease.23-26 However, patients with CNS involvement at relapse (occurring in almost 1 of 3 of relapses in high-risk DLBCL27) are frequently excluded from trials of novel agents and cellular therapies, and their prognosis is extremely poor (median OS 5-6 months).5
There is no broad consensus worldwide regarding how best to reduce the risk of CNS relapse.28 HD-MTX has been widely adopted as CNS prophylaxis in DLBCL, with initial supporting evidence derived from studies demonstrating efficacy in the treatment of primary CNS lymphoma.29 Historical, retrospective, nonrandomized studies also suggested a benefit of HD-MTX in DLBCL patients at high risk of CNS relapse, either intercalated with R-CHOP14 or delivered at EOT.13 Recently, large retrospective analyses have demonstrated no apparent benefit of HD-MTX in the reduction in CNS relapse risk.18,19 Patients at the highest risk of CNS relapse are also those at greatest risk of systemic treatment failure, and therefore there has been a lack of agreement about how HD-MTX should be incorporated alongside R-CHOP, with the risk of early CNS progression balanced against the risk of interrupting systemic treatment. Our previous UK study demonstrated increased delays to R-CHOP with i-HD-MTX compared with EOT, but the number of CNS relapse events was too small to conclude that the approaches were equivalent in efficacy.20
To our knowledge, this international, multicenter collaboration represents the largest dataset of patients with DLBCL receiving HD-MTX as CNS prophylaxis. The study achieved its primary endpoint of demonstrating noninferiority of EOT HD-MTX compared with i-HD-MTX with regards to CNS relapse risk. This finding was observed despite an increased cumulative HD-MTX dosage in i-HD-MTX compared with EOT patients. When identifying these patients retrospectively, there is a risk that some patients planned for EOT HD-MTX are missed due to early progression. Indeed, the inferior PFS and OS in the i-HD-MTX group suggest this. To address this, we performed a landmark analysis assessing only those patients alive and progression-free at 6 months. This included 90.5% of patients and again demonstrated noninferiority and, importantly, no PFS/OS difference.
The proportion of CNS-IPI 4 to 6 patients in our study was relatively low (44%). However, the CNS-IPI is an imperfect tool, with a high-risk score resulting in a positive predictive value of only 12%. Other established, independent risk factors include specific EN site involvement (eg, testicular, renal/adrenal, and breast) and the total number of EN sites involved. We performed analyses aimed at determining whether the timing of HD-MTX delivery had any influence on CNS relapse in the most high-risk patients. Again, differences were small, though we acknowledge restricting analyses to small subgroups may result in small differences between groups being missed. However, we could still exclude a 5% difference for the composite high-risk group (absolute difference +0.2%), and, although not quite excluded for the high CNS-IPI group, the absolute difference favored EOT (−0.7%) and the upper confidence interval only just crossed +5% (+5.4%).
Much of the literature addressing CNS relapse in DLBCL does not distinguish between isolated CNS relapse and CNS relapse occurring either with or after systemic progression. Indeed, Schmitz and colleagues do not give this detail.6 Arguably, any CNS relapse occurring concurrently with or after systemic relapse represents a failure of systemic therapy, with the aim of prophylactic HD-MTX being purely to prevent isolated CNS events. A recent retrospective analysis (n = 226) reported a significant reduction in isolated CNS relapses with HD-MTX but no difference in OS or concomitant CNS-systemic relapses.30 We excluded any CNS relapse occurring after the first systemic DLBCL relapse/progression and recorded data on whether the CNS relapse was isolated. Considering that isolated CNS relapses are likely to occur because of occult clones taking sanctuary in the CNS either at diagnosis or early in the disease course, there is a theoretical rationale that early HD-MTX delivery may be important. However, in the 73.1% of cases where CNS relapse was isolated, we found no benefit for i-HD-MTX.
We demonstrate that i-HD-MTX significantly increases the risk of R-CHOP delay, with 19% of i-HD-MTX treatments resulting in a delay to subsequent R-CHOP and 26% of patients in the i-HD-MTX group experiencing ≥1 delay of ≥7 days during therapy vs 13% in the EOT cohort, though we acknowledge that some patients planned for EOT HD-MTX who suffered complications and R-CHOP delays may have had HD-MTX omitted, and therefore are not captured in this study. Given the need to maintain relative dose intensity in DLBCL, these delays are clinically relevant, especially in patients inherently at high risk of systemic treatment failure. We found that increasing age was an independent risk factor for delays with i-HD-MTX, suggesting i-HD-MTX should be used with particular caution in older patients, though our repeated measures analysis suggested that earlier delivery (before day 10) may be associated with a lower risk of delay. Although we found no clear evidence of an increase in risk by dose, R-CHOP cycle number, or HD-MTX dose number, HD-MTX delivery was decided by site and may have been guided by the deliverability of previous cycles, possibly biasing our data. To understand these relationships, an analysis based on patients treated on 1 protocol is needed.
Direct comparison of HD-MTX toxicity between i-HD-MTX and EOT approaches is problematic, as some of the toxicities with i-HD-MTX may be influenced by concurrent R-CHOP. We were unable to record toxicities between R-CHOP cycles in the EOT group to serve as the most accurate comparator. However, the observed rates of febrile neutropenia, mucositis, and renal toxicity (all 15% to 17%) associated with i-HD-MTX are of concern, particularly when the benefit is questionable.
Concurrent IT therapy was used in a significant proportion of patients, particularly in the EOT group, likely due to clinician concern that some form of CNS-directed therapy should be delivered early. However, there is cumulative data to suggest that IT therapy is ineffective in reducing CNS relapses in DLBCL, including a large systematic review of over 7000 DLBCL patients, which demonstrated no benefit of standalone IT therapy in preventing CNS relapse.10 We demonstrate that the use of concurrent IT prophylaxis was not associated with a reduction in CNS relapse on multivariable analysis, and there was no evidence of an interaction with HD-MTX timing. However, all patients were given HD-MTX, and therefore we were unable to assess whether IT prophylaxis without HD-MTX shows benefit.
The overall rate of CNS relapse observed raises concern about any potential efficacy of HD-MTX, irrespective of delivery timing. The observed overall 3-year rate of 5.7% was only marginally less than the predicted risk of 7% when the CNS-IPI risk model was applied to our cohort. Furthermore, our 3-year cumulative incidence of CNS relapse in high CNS-IPI patients was 9.1%, which is almost identical to that observed in the original CNS-IPI study, where no systemic HD-MTX was used in the design cohort and very few in the validation cohort.6 Recent retrospective analyses demonstrate no apparent benefit of HD-MTX prophylaxis,15-17 including a multicenter analysis of approximately 2300 high-risk patients, which found no difference in CNS relapse between patients receiving HD-MTX vs not.19 Furthermore, the overall rate of CNS relapse of 9% in the latter study, which included 1890 patients receiving no HD-MTX, was identical to the rate observed in patients with CNS-IPI 4-6 in our analysis.
To answer the question of HD-MTX efficacy definitively, a randomized controlled trial of HD-MTX vs no prophylaxis is required, but sample size would present significant logistical challenges. Our data, in conjunction with other recent literature, suggest a limited benefit for HD-MTX for the majority of DLBCL patients, irrespective of the timing of delivery. However, even the large Lewis and colleagues analysis is limited in its ability to exclude the benefit of HD-MTX in the highest risk subgroups, such as those with CNS-IPI 6 or with high-risk EN site involvement (eg, testicular and breast). There is also prospective data to suggest a benefit of HD-MTX for patients with testicular DLBCL, with recently presented results from the IELSG30 trial demonstrating no CNS relapses following IV and IT CNS prophylaxis.31
To date, no other agent has been shown to reduce the risk of CNS relapse in DLBCL. Novel agents, such as ibrutinib and lenalidomide, have been proposed as potential agents capable of influencing CNS relapse risk due to their ability to cross the blood-brain barrier. Although both agents have shown promising activity in primary and secondary CNS involvement with B-cell malignancies, neither have shown overall benefit for patients with DLBCL when incorporated into R-CHOP in large prospective trials.32,33 Whether these drugs could specifically benefit the small subset of patients at most risk of CNS relapse remains an unanswered question. Until a more effective prophylactic strategy is demonstrated, some may still reasonably choose to use HD-MTX for the most high-risk patients, and we provide valuable data to support decision-making around its delivery.
The strengths of this study are the multicenter design, large sample size, preplanned power calculation, and the granularity of data, particularly with regards to HD-MTX delivery and CNS relapse. The main limitations are those inherent to retrospective, nonrandomized observational analyses, with potential for selection bias and imbalance between treatment groups, in particular, the immortal time bias for EOT patients due to the lack of recorded data on “intention-to-treat with EOT HD-MTX.” The EOT cohort could not, by definition, have experienced an event during therapy and remained fit to receive HD-MTX at this point. This may have excluded frailer patients who experienced delays during immunochemotherapy. However, both groups were extremely well balanced for baseline characteristics, with all analyses of relapse and survival including adjusted models to account for potential imbalances, and importantly, our results held within the landmark cohort, who should not be prone to immortal time bias. The selection criteria for CNS prophylaxis varied between centers, reflecting the limited evidence to guide such decisions, particularly before the introduction of the CNS-IPI. Only 50% of patients had baseline CNS evaluation, which introduces a potential risk of selection bias and of including patients with occult CNS involvement at diagnosis.
In conclusion, in an international cohort of 1384 patients, we demonstrate that delivery of EOT HD-MTX did not increase the risk of CNS relapse compared with early integration during R-CHOP/R-CHOP-like therapy. The CNS relapse rate observed in high-risk patients in our study was relatively high despite the use of HD-MTX, raising further concern about the efficacy of HD-MTX as CNS prophylaxis. We cannot conclude from our data that HD-MTX, intercalated or not, does not benefit a small subset of very high-risk patients, although we recognize that usage is likely to decrease substantially in light of the recently presented and published data. In the selected patients where HD-MTX may still be considered, we provide data to support EOT delivery for most patients. i-HD-MTX should be used with caution in older patients or those at increased risk of toxicity, and if employed, the HD-MTX should be delivered earlier in the R-CHOP cycle (prior to day 10) to reduce R-CHOP delays. It may be that investigating the incorporation of novel agents and using more sophisticated techniques (eg, CSF ctDNA) to identify high-risk patients are areas where the field should focus attention.
Acknowledgments
The authors thank the following health care professionals for their expert dedication to data collection: Catherine Thieblemont (Hôpital Saint-Louis, Assistance Publique-Hôpitaux de Paris), Sridhar Chaganti (University Hospitals Birmingham), George Follows (Cambridge University Hospitals NHS Foundation Trust), Anca Prica (Princess Margaret Cancer Centre), Adam Olszewski (Brown University and Lifespan Cancer Institute), Barbara Botto (AOU Città della Salute e della Scienza di Torino), Corinne Haioun (Hospital Henri Mondor), Caroline Besson (Centre Hospitalier de Versailles), Olivier Tournilhac (Service d'Hématologie et de Thérapie Cellulaire, CHU Estaing, Université Clermont Auvergne), Pietro Di Ciaccio (St Vincent's Hospital Sydney), Agnes Olivrie and Julie Abraham (Hématologie Clinique et Thérapie Cellulaire, CHU de Limoges), Dipti Talaulikar and Caitlin Coombes (Australian National University and Canberra Health Services), Raul Cordoba (Fundacion Jimenez Diaz University Hospital, Health Research Institute), Adolgo de la Fuente (MD Anderson, Madrid, Spain), Rebecca Oliver and Laura Percy (University Hospitals Bristol NHS Foundation Trust), Kamel Laribi and Catherine Truong (Centre Hospitalier Le Mans, Le Mans), Ruth Clifford (University Hospital Limerick), Jordan Carter and Andrew Evens (Rutgers Cancer Institute), Brian Henessy (University Hospital Waterford), Wendy Osborne and Thomas Creasey (Newcastle Hospitals NHS Foundation Trust), and Javier Penalver and Maria Garcia Roa (Hospital Universitario Fundacion Alcorcon).
Authorship
Contribution: M.R.W., T.A.E., A.A.K., K.C., and P.M. designed the study, analyzed data, and wrote the paper; A.A.K. performed all statistical analyses; and all authors participated in the collection of data and writing/reviewing the manuscript.
Conflict-of-interest disclosure: M.R.W. received conference fees from Takeda, Janssen, and Kite/Gilead; honoraria from Abbvie and Kite/Gilead. T.A.E. received honoraria from Roche, Kite/Gilead, Janssen, Abbvie, AstraZeneca, Loxo Oncology, Beigene, and Secura Bio; provided consultancy for Roche, Abbvie, Loxo Oncology, Incyte, Secura Bio. M.A. received honoraria from Takeda and Roche; research funding from Pfizer. E.S. received honoraria from Riemser Pharma GmbH; research funding from Roche and Abbvie. M.K. provided consultancy to Roche, Antegene, and Genor Biopharma. M.N. received research funding from TG Therapeutics, Genmab, Genentech/Roche, and Gilead. K.L.L. received honoraria from AstraZeneca, Janssen, and Roche; patents and royalties from Janssen and Novartis; provided consultancy to AstraZeneca. A.K.Ø. received travel expenses from Abbvie. A.S. received honoraria from Janssen. N.S. received honoraria and membership on an entity's board of directors or advisory committees for Abbvie, Janssen, and Roche. L. Roulin received travel expenses from Janssen. K.M. received travel and meeting expenses from Bristol-Myers Squibb. N.H. holds membership on an entity's board of directors or advisory committees and speakers bureau for Novartis. A.G. received speaker honoraria from Roche, Janssen, Abbvie, Celgene, Fresenius, and Novo Nordisk; travel and accommodation expenses from Roche, Janssen, and Abbvie. T.C.E. ended employment in the past 24 months with Roche; received speaker fee from Abbvie. C.Y.C. provided consultancy, received honoraria and other (advisory) for/from Roche, Janssen, MSD, Gilead, Ascentage Pharma, Beigene, AstraZeneca, Loxo/Lilly, and TG Therapeutics; received research funding from Abbvie and Celgene. A.J.M.F. has membership on an entity's board of directors or advisory committees for Gilead, Novartis, Juno, PletixaPharm, Roche, and Incyte; received research funding from BMS, Beigene, Pharmacyclics, Hutchison Medipharma, Amgen, Genmab, ADC Therapeutics, Gilead, Novartis, and Pfizer. C.P.F. received honoraria and has membership on an entity's board of directors or advisory committees and received research funding for/from Roche; received speaker fees from Janssen. K.C. provided consultancy and received travel expenses to scientific conferences and speakers bureau for/from Roche, Janssen, Kite/Gilead, and Takeda; provided consultancy and speakers bureau for Gilead and Incyte; provided consultancy for Celgene and Atara; received travel expenses to scientific conferences for BMS/Celgene. P.M. received honoraria and has membership on an entity's board of directors or advisory committees from/for Roche, Kite, Takeda, and Beigene; received travel support from Gilead and Janssen. The remaining authors declare no competing financial interests.
Matthew R. Wilson, Department of Haematology, Beatson West of Scotland Cancer Centre, 1053 Great Western Rd, Glasgow G12 0YN, United Kingdom; e-mail: matthew.wilson@ggc.scot.nhs.uk.
REFERENCES
Author notes
These data were presented at the 63rd Annual Meeting of the American Society of Hematology, 11-14 December 2021.
Qualified researchers may request data from the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
In a large, multicenter retrospective analysis of IV methotrexate (MTX) during frontline treatment for diffuse large B-cell lymphoma (DLBCL), Wilson et al report that high dose intravenous MTX given at the end of treatment was as effective and less toxic for central nervous system (CNS) prophylaxis than MTX given during chemoimmunotherapy. Furthermore, the overall rate of CNS relapse in patients receiving CNS prophylaxis was similar to that in patients not receiving intensive prophylaxis. These data call into question the selection of patients for CNS prophylaxis with intravenous MTX.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal