In this issue of Blood, Bennett et al describe thrombopoiesis as a 3-step process and identify cytokine receptor-like factor 3 (CRLF3) as a novel regulator of preplatelet maturation, which might serve as a potential therapeutic target for the treatment of essential thrombocythemia (ET).1
Platelet production from mature megakaryocytes (MKs) has long been divided into 2 distinct phases: (1) the differentiation of MKs from hematopoietic progenitors in the bone marrow and (2) the formation of proplatelet protrusions through the sinusoidal endothelium and the subsequent release of platelets into the bloodstream. Investigations have focused on the identification of mechanisms of proplatelet elongation and release, whereas the importance of platelet maturation in circulation has not been well studied. Previous studies have already identified several platelet intermediates ranging from barbell-shaped or multibody proplatelets that are severed into platelets within circulation to circular preplatelets with increased width.2,3 Here, Bennett et al describe preplatelet maturation within the bloodstream as the third step of platelet production, which is dependent on the orphan cytokine receptor-like factor CRLF3, that, albeit recently being identified as a neuroprotective erythropoietin receptor in locusts, has not heretofore been investigated in mammals.4
ET is caused by clonal proliferation of hematopoietic progenitors carrying mutations within the thrombopoietin receptor Mpl, its downstream effector Janus kinase 2, or calreticulin. Patients have markedly elevated platelet counts associated with an increased risk of thrombotic events. Current treatment options are limited to inhibitors of platelet functions (aspirin) or production (hydroxyurea, anagrelide).5 In addition to identifying CRLF3-dependent maturation of preplatelets, Bennett et al proposed inhibition of CRLF3 as a novel approach to lower platelet counts in ET and explored this idea in a mouse model of ET.
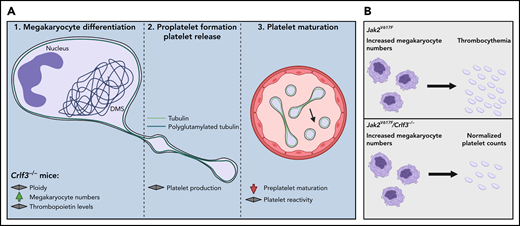
To identify the role of CRLF3 in megakaryo- and thrombopoiesis, the authors used a CRLF3-deficient mouse line, that had an isolated reduction in platelet counts of 25% to 48%.1 Despite an increased number of MKs in the bone marrow, thrombopoietin levels were normal. CRLF3-deficient MKs matured normally with largely unaltered proplatelet formation in vitro and in vivo. However, blood smears and transmission electron micrographs revealed an increased number of immature preplatelets in CRLF3-deficient mice, which were normalized after splenectomy. Although platelet maturation appeared to be affected by the loss of CRLF3, platelet reactivity and function were unaltered, as assessed by adhesion assays and flow cytometry in vitro and an in vivo model of thrombus formation. The authors identify enhanced polyglutamylation of tubulins in MKs that is conferred to a hyperstability of microtubules in platelets (see figure panel A). Among other cytoskeletal proteins, they discover a novel interaction of CRLF3 with the Hippo pathway proteins Stk38 and, indirectly, Mob1, by performing mass spectrometry on human-induced pluripotent stem cell–derived MKs, thus suggesting that the Hippo pathway is important for preplatelet maturation. To corroborate their data, the authors used imputed genotype data from European ancestry participants and identified genetic variants within CRLF3, STK38, and MOB1 to be associated with platelet distribution width implying their link to thrombopoiesis in humans. Last, the authors provide evidence that targeting CRLF3 is beneficial to lower platelet counts in a mouse model of ET (see figure panel B).
The third step of thrombopoiesis is defective upon lack of CRLF3 in mice. (A) According to Bennet et al, platelet generation is dependent on 3 distinct stages: Megakaryocyte (MK) differentiation from hematopoietic progenitors (1), proplatelet formation (2), and the maturation of preplatelets to platelets within the circulation (3). Loss of CRLF3 results in thrombocytopenia despite unaltered MK differentiation and proplatelet formation owing to impaired preplatelet maturation within circulation. (B) Deletion of CRLF3 in a mouse model of essential thrombocythemia (ET) (Jak2V617F) decreases platelet counts and improves disease outcome. DMS, demarcation membrane system. Image was created using Biorender.com.
The third step of thrombopoiesis is defective upon lack of CRLF3 in mice. (A) According to Bennet et al, platelet generation is dependent on 3 distinct stages: Megakaryocyte (MK) differentiation from hematopoietic progenitors (1), proplatelet formation (2), and the maturation of preplatelets to platelets within the circulation (3). Loss of CRLF3 results in thrombocytopenia despite unaltered MK differentiation and proplatelet formation owing to impaired preplatelet maturation within circulation. (B) Deletion of CRLF3 in a mouse model of essential thrombocythemia (ET) (Jak2V617F) decreases platelet counts and improves disease outcome. DMS, demarcation membrane system. Image was created using Biorender.com.
Although the presence of preplatelets and their severing dynamics within the circulation have been investigated,3,6 so far, no molecular switches regulating preplatelet maturation have been identified. The mouse model described by Bennett et al might therefore be a useful tool to gain insights into the distinct intermediate stages that precede mature platelets. Recent data challenged the classical model of proplatelet formation as the main mechanism of platelet release, suggesting that membrane budding rather than proplatelet elongation and release delivers platelets into the bloodstream.7 However, the possibility of microvesicles budding off from MKs in this context was not investigated sufficiently, thus leaving the hypothesis of platelet budding unresolved.8 In line with this, the present study provides evidence for a significant contribution of preplatelets to the overall platelet pool, thus further supporting the hypothesis that proplatelet formation contributes to the majority of platelets in vivo.
Advances in imaging and sequencing techniques have identified a variety of molecular players and posttranslational modifications on cytoskeletal proteins that are essential to drive protrusion formation and proplatelet elongation. Dynamic regulation of polyglutamylation and acetylation of α- and β1-tubulin were previously shown to be essential for proplatelet release and platelet functionality.9,10 This was partially supported in the present study by Bennett et al, in which aberrant polyglutamylation presumably increases microtubule stability in MKs. However, because no differences in posttranslational modifications, notwithstanding the enhanced microtubule stability, were observed in CRLF3-deficient (pre)platelets, additional mechanisms appear to be causative for their impaired severing.
Although the study by Bennett et al provides new insights into a so-far unknown signaling pathway important for preplatelet maturation, several questions need to be addressed. Targeting preplatelets to normalize elevated platelet counts in a mouse model of ET might help prevent a thrombotic event. In contrast, it would not impact progression of the disease into myelofibrosis because MK numbers in the bone marrow were still elevated upon loss of CRLF3. Moreover, the authors base their idea of targeting CRLF3 on the findings that preplatelets are cleared in the spleen, although platelet clearance mechanisms may differ between humans and mice. Future studies are necessary to identify other molecular drivers of preplatelet maturation to extend our knowledge on this stage of platelet generation and to investigate whether they can be targeted therapeutically.
Conflict-of-interest disclosure: J.E.I. has financial interest in and is a founder of PlateletBio, a biotechnology company focused on making donor-independent platelet-like cells at scale. The interests of J.E.I. are managed by Boston Children's Hospital. I.C.B. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal