In this issue of Blood, Wu et al1 describe a novel yet critical transcriptional factor network that confers resistance to immunomodulatory imide drugs (IMiDs) in T-cell lymphomas (TCLs). They identify a pivotal codependence of Ikaros (IKZF1) and zinc finger protein 91 (ZFP91) in IMiD-resistant TCLs, thereby revealing an acquired mechanism of resistance to IMiD treatment. Furthermore, their study provides an attractive therapeutic strategy that uses more potent next-generation degraders targeting IKZF1 and ZFP91 for treating patients with TCL.
TCLs are a group of heterogeneous lymphoid malignancies of T-cell origin with diverse clinical presentations and therapeutic responses. Many TCLs have a poor prognosis with a <30% 5-year overall survival in some subtypes.2 To date, 29 different subtypes have been recognized by the World Health Organization on the basis of their histological and molecular features.3 Therapy for TCLs, however, has generally been extrapolated from trials of B-cell lymphomas that use multiagent chemotherapy such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Given the poor outcomes with standard therapy, there remains a dire need to identify novel vulnerabilities for effective treatment strategies in patients with TCL.
IMiDs, principally thalidomide and its derivatives lenalidomide and pomalidomide, have received approval by the US Food and Drug Administration to treat patients with multiple myeloma or mantle cell lymphoma. IMiDs bind to cereblon (CRBN), which is involved in the formation of a ubiquitination ligase complex, and further promote the selective degradation of specific substrates, notably the Ikaros family transcription factor IKZF1.4 The IKZF family of transcription factors plays a crucial role in the differentiation and development of immune cell populations.5 Mutations and copy number alterations affecting the IKZF1 locus have been reported in several hematologic malignancies, including myeloproliferative neoplasms6 and B-cell7 and T-cell acute lymphoblastic leukemia.8 However, our understanding of the role and function of IKZF1 in TCLs is very limited. Even though IKZF1 has been identified as a vulnerability in some TCL-derived cell lines by CRISPR/Cas9 screens,9 suggesting that IMiDs should be able to target IKZF1 in TCLs, IMiDs have shown disappointing activity in TCLs.10 There are still gaps in understanding the mechanisms underlying TCL resistance against treatment with IMiDs.
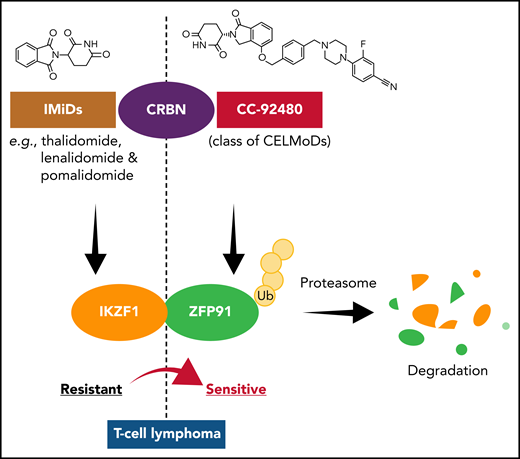
In the article by Wu et al, elegant and robust in vitro and in vivo experimental approaches in multiple TCL lines and patient-derived xenografts demonstrated that selective resistance to IMiDs in TCLs involves codependence of IKZF1 and ZFP91. They also addressed the question of whether resistance to IMiDs is simply a result of pharmacologic failure or a lack of dependence on the identified target proteins IKZF1 and ZFP91. They found a close correlation between the CRBN expression levels and the extent of IMiD-induced IKZF1 degradation. In addition, when CRBN overexpression was induced in IMiD-resistant TCL cells, the degree of IKZF1 and ZFP91 degradation was markedly increased, and the cells were re-sensitized to treatment with IMiDs. On the basis of these results, the investigators hypothesized that limited CRBN expression reduces IMiD activity in TCLs and therefore, it is highly likely that a more potent degrader can overcome IMiD resistance in TCLs. They then explored the efficacy and clinical potential of CC-92480, an agent from a new class of CRBN E3 ligase-modulating drugs that share the same glutarimide rings with IMiDs for CRBN- and substrate-binding in IMiD-resistant TCLs in vitro and in vivo. CC-92480 was broadly effective against multiple TCL subtypes and showed higher potency than IMiDs by an ∼100-fold lower concentration with selective yet robust degradation of IKZF1 and ZFP91 across IMiD-resistant TCL lines. Moreover, using preclinical models of several subtypes of TCL patient-derived xenografts, CC-92480 induced tumor regression and prolonged survival in vivo. Thus, CC-92480 overcame IMiD resistance in TCLs by targeting IKZF1 and ZFP91, which suggests that this novel class of agents shows a good therapeutic window for clinical use in patients with TCLs.
Overall, the results presented by Wu et al expand our understanding of IMiD resistance mechanisms in TCLs (see figure) and show the potential of the novel agent CC-92480 in overcoming IMiD resistance in TCLs. However, the clinical safety and efficacy of this agent is currently being investigated only in trials in multiple myelomas but not in TCLs. Considering the important insights regarding disease biology revealed by this study, more studies using IMiDs to investigate prospects for improving outcome of patients affected by TCLs are certainly warranted.
CC-92480, a cereblon (CRBN) E3 ligase-modulating drug (CELMoD), overcomes IMiD resistance in T-cell lymphomas. IMiD-resistant T-cell lymphomas show crucial co-dependence of IKZF1 and ZFP91 transcription factors. Binding of CC-92480 to CRBN leads to selective and potent degradation of IKZF1 and ZFP91, overcoming IMiD resistance in multiple subtypes of T-cell lymphoma. Ub, ubiquitin.
CC-92480, a cereblon (CRBN) E3 ligase-modulating drug (CELMoD), overcomes IMiD resistance in T-cell lymphomas. IMiD-resistant T-cell lymphomas show crucial co-dependence of IKZF1 and ZFP91 transcription factors. Binding of CC-92480 to CRBN leads to selective and potent degradation of IKZF1 and ZFP91, overcoming IMiD resistance in multiple subtypes of T-cell lymphoma. Ub, ubiquitin.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal