In this issue of Blood, Loeffler et al1 provide evidence that human hematopoietic stem cells (hHSCs) can undergo asymmetric cell division (ACD) to generate 2 cells with distinct functional fates, and that lysosome asymmetric inheritance determines these fates. These findings answer the long-standing question of ACD in HSC biology and uncover unexpected factors that determine cell fate that may be used for developing new protocols for HSC expansion.
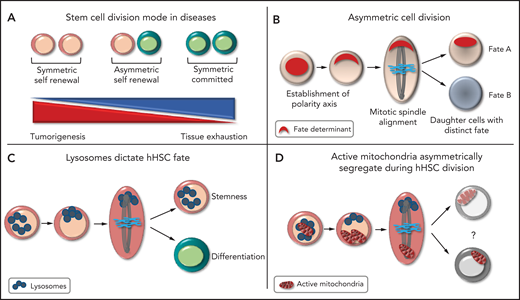
Remaining a stem cell or committing to differentiation is the most important fate-determining event in the lifetime of the HSC. This process maintains the balance between the pool of HSCs and progenitors and ensures homeostasis of the hematopoietic system. During development, stem cells use ACD to create cellular diversity and maintain adequate numbers of both stem cells and differentiated cells. Stem cells can adapt their mode of division and divide symmetrically (generating 2 stem cells or 2 progenitors) or asymmetrically, to meet the regenerative need of tissues,2 the failure of which can cause long-term tissue exhaustion or tumor development (see figure panel A). Although hHSC are believed to divide asymmetrically, definite evidence has been lacking.
The principles of asymmetric cell division. (A) Different modes of cell division. (B) Steps leading to the asymmetric partitioning of fate-determining factors. (C) Asymmetric inheritance of lysosomes is linked to asymmetric hHSC fate. (D) Daughter cells receiving more lysosomes inherit fewer active mitochondria in hHSCs, the function of which is unknown.
The principles of asymmetric cell division. (A) Different modes of cell division. (B) Steps leading to the asymmetric partitioning of fate-determining factors. (C) Asymmetric inheritance of lysosomes is linked to asymmetric hHSC fate. (D) Daughter cells receiving more lysosomes inherit fewer active mitochondria in hHSCs, the function of which is unknown.
ACD is the unequal partitioning of cellular components during cell division that enables daughter cells to have distinct fates. ACD is controlled by the asymmetric reorganization of the cytoskeleton, which results in cellular polarity, with an asymmetric accumulation of factors that determine the fate of the cell. The orientation of the mitotic spindle along the polarity axis ensures unequal partitioning of these factors between daughter cells, with the level of fate-determining factors received deciding the future identity of each daughter cell (see figure panel B).2 Demonstrating ACD requires the linking of factors that alter cell fate to their asymmetric distribution. Identifying these linking factors has been a challenging task in HSCs because of the limited knowledge of the factors that determine HSC identity. HSCs are retrospectively defined by their ability to generate mature cells, making assessment of HSC fate dependent on the behavior of the progeny. ACD was first suggested in HSCs when paired daughter cells generated clones of distinct size and myeloid lineage potential in vitro.3 In murine HSCs, cellular factors, including lysosomes, can asymmetrically segregate and alter HSC fate.4,5 In hHSCs, studies have shown that the surface markers CD53 and CD62L are unequally partitioned, which can correlate with the potential lineage of daughter cells.6 The endosomal protein Ap2a27 and myosin II8 can both alter hHSC fate and may be asymmetrically inherited. None of the aforementioned studies linked asymmetric inheritance of these components to hHSC fate.
In a tour de force, Loeffler et al were able to link asymmetric lysosomal inheritance to hHSC fate ex vivo, demonstrating that hHSCs can use ACD to generate daughter cells with distinct fates. They used a long-term quantitative single-cell imaging technique to quantify factors inherited by paired daughter cells during hHSC division and to trace the offspring of each daughter cell until the potential lineage was revealed. In a highly purified CD49f+ hHSC population, they first showed that lysosomes are asymmetrically inherited during hHSC division. Importantly, asymmetric lysosomal inheritance correlates with asymmetric segregation of different stem cell markers. The group created real-time differentiation landscapes of different cellular states during culture, by using surface markers enriched for stem cell potential (CD201 and CD49c). Cells receiving more lysosomes preferentially produced cells expressing high levels of CD49c that divided slower, an indicator of stem cell potential. Daughter cells receiving fewer lysosomes were prone to give rise to CD33-expressing cells, indicative of myeloid differentiation. Hence, the absolute quantity of inherited lysosomes correlated with daughter cell fate, predicting the cell cycle length and differentiation potential of the daughter cells in vitro (see figure panel C). It will be interesting to confirm that hHSCs receiving more lysosomes retain long-term repopulating activity in vivo. Loeffler et al observed other types of hHSC division, implying that ACD is regulated and can be modulated to meet the demand. ACD is most likely not limited to lysosome distribution. They showed that endosomes, active mitochondria, and mitophagosomes can all be asymmetrically partitioned. Interestingly, daughter cells receiving more lysosomes inherited fewer active mitochondria during hHSC division, although the impact on hHSC fate was less clear (see figure panel D), perhaps because mitochondria are known to permanently remodel during and after HSC division.9 These findings imply that lysosomes are bona fide hHSC fate-determining factors and that organelles and metabolic functions are integral parts of ACD in hHSCs.
Although these findings provide remarkable insights into one of the biggest mysteries of stem cell biology, much remains to be learned about the regulatory pathways that determine how stemness is inherited by daughter cells. How are lysosomes asymmetrically partitioned? How do lysosomes dictate HSC fate? Lysosomes are major components of the cellular degradative machinery, but they also serve as signaling hubs for anabolic processes and thus have both progrowth and antigrowth functions. HSCs possess large lysosomes that degrade slowly and act as reservoirs of cargo, including mitochondria, to maintain HSC quiescence.10 It is possible that lysosomes terminate the activity of HSCs to enable daughter cells to return to quiescence. Conversely, do lysosomes provide building blocks for biomass and progrowth signals for HSC activation? If so, how is lysosomal activity regulated throughout HSC division? Likewise, the exact role of active mitochondria in hHSC fate requires further investigation.
Ingeneral, there is a need to examine HSC behavior and understand organelle biology and metabolic requirements during active HSC division when fate is determined. The development of the metabolomics of a small number of cells integrated with sophisticated RNA sequencing and assay for transposase-accessible chromatin sequencing at the single-cell level will help in dissecting the regulatory pathways of ACD in HSCs. Influencing HSC division modes can be a powerful way to amplify HSCs, as it would enable doing so in only a few divisions. This knowledge will guide the development of culture protocols for HSC-based therapies. Future research should examine how HSCs use ACD, whether ACD contributes to HSC heterogeneity, and how ACD is modulated in response to stress or contributes to clonal hematopoiesis and hematopoietic neoplasms if deregulated. Answering these questions is critical to exploiting the regenerative potential of HSCs for clinical purposes or for antitumor therapies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

Comments
Asymmetric cell divisions in the hematopoietic system
Pluripotent, very small embryonic-like stem cells (VSELs) are most primitive stem cells, sit at top of hierarchy, and give rise to HSCs [2]. VSELs (2-6 µm) undergo ACD whereby they divide to self-renew and generate bigger HSCs (>6 µm). VSELs undergo distinct epigenetic changes while undergoing ACD and majority of chromatin gets inactivated in the HSCs which become lineage-restricted and fate committed. VSELs express nuclear OCT-4 and NUMB is selectively expressed only in HSCs during ACD[3]. ACD/SCD and clonal expansion of stem cells in the mouse bone marrow 48h after FSH treatment has also been delineated [4]. Similar ACD/SCD has been reported in mice testes, uterus and ovaries by our group.
Data is now available reporting increased expression of OCT-4 in various kinds of leukemia Elevated expression of SALL4 in CML patients has also been reported especially in patients in chronic phase. Both nuclear OCT-4 and SALL4 are specific pluripotent markers of VSELs. Cancer in various reproductive tissues occurs due to excessive self-renewal of OCT-4 positive VSELs and their blocked differentiation [5]. Greater insight into ACD is essential to gain insights into elusive leukemic stem cells that cause recurrence.
References
1. Filippi M-D. Asymmetric division: the choice of fate for huHSCs. Blood. 2022; 139(13): 1930–1932. doi: 10.1182/blood.2021012726
2. Ratajczak MZ, Ratajczak J, Kucia M. Very small embryonic-like stem cells (VSELs). Circ Res. 2019;124(2):208-210. doi: 10.1161/CIRCRESAHA.118.314287.
3. Ganguly R, Metkari S, Bhartiya D. Dynamics of bone marrow VSELs and HSCs in response to treatment with gonadotropin and steroid hormones, during pregnancy and evidence to support their asymmetric/symmetric cell divisions. Stem Cell Rev Rep. 2018;14(1):110-124. doi: 10.1007/s12015-017-9781-x.
4. Bhartiya D, Kaushik A, Singh P, Sharma D. Cancer initiates due to excessive self-renewal and blocked differentiation of tissue-resident, OCT-4 positive VSELs. Stem Cell Rev Rep. 2022. doi: 10.1007/s12015-022-10424-x.
5. Bhartiya D, Patel H, Kaushik A, Singh P, Sharma D. Endogenous, tissue-resident stem/progenitor cells in gonads and bone marrow express FSHR and respond to FSH via FSHR-3. J Ovarian Res. 2021;14(1):145. doi: 10.1186/s13048-021-00883-0.