In this issue of Blood, Revollo et al1 expand our understanding of primary hemostasis by demonstrating the crucial role of vertebrate lonesome kinase (VLK; PKDCC) in platelet function. VLK is a secreted kinase that regulates substrate phosphorylation along the secretory pathway and in the extracellular space.
Many extracellular proteins in blood and tissue are phosphorylated,2 but only a few kinases with extracellular functions have been identified. Tyrosine phosphorylation of secreted proteins or extracellular domains of transmembrane proteins seems to be executed primarily by VLK.3 As its name indicates, this kinase occurs only in vertebrates, where it is well conserved across different species.4 VLK is required for lung development and ossification, and its crucial role in skeletal development has been confirmed in humans.5 Full-length VLK has a signal peptide that targets the kinase to the endoplasmic reticulum and, thereby, the secretory route.3,6 It is capable of phosphorylating extracellular matrix components.7 However, VLK differs significantly from other kinases, and its exact roles in the secretory pathway or in the extracellular space are still unclear. Strikingly, VLK is very abundant in platelets, where it is found predominantly in α-granules and is secreted after platelet activation. The functional role of VLK in platelets is now revealed by Revollo et al,1 who show by mass spectroscopy that platelet activation via protease activating receptor 1 leads to a pronounced upregulation of protein tyrosine phosphorylation. Four of 213 unique phosphopeptides mapped to proteins with signal peptides or extracellular domains. The investigators also showed that 1 of these proteins (ENTPD6) is a substrate of VLK and, thus, may be a potential candidate to modulate platelet activation.8 Furthermore, the 3 additional secreted tyrosine-phosphorylated proteins have functions in platelet biology and coagulation.
To investigate the role of VLK in vivo, the investigators generated a mouse model with a megakaryocyte/platelet-specific deletion of the kinase. Although there were no obvious changes in platelet morphology, counts, or contents, the lack of VLK resulted in an ∼50% decrease in protein tyrosine phosphorylation in resting and activated platelets. This included proteins secreted from platelets upon degranulation, such as thrombospondin-1. Testing the function of VLK-deficient platelets in vitro revealed impaired aggregation under submaximal activation conditions, whereas higher doses of agonists resulted in a normal response. Strikingly, release of dense granules and, thus, adenosine diphosphate (ADP)/adenosine triphosphate (ATP) was completely abolished in platelets lacking VLK, implying that the amplification loop utilizing ADP/ATP and purinergic receptors is dysfunctional in the knockout mice. Similarly, Akt and ERK phosphorylation, important signaling intermediates in platelet activation, were decreased in VLK-knockout platelets. Both were restored by the external addition of ADP, as was platelet aggregation.
The investigators further demonstrate a crucial role for VLK in thrombus formation in vivo. Aggregation of VLK-deficient platelets was nearly abolished in a model of cremaster arteriole laser injury, and fibrin deposition was significantly reduced in comparison with control mice. However, tail bleeding assays did not show any VLK-specific differences, indicating that thrombus formation is reduced in the absence of VLK, but that overall hemostasis is not substantially affected. Consequently, VLK inhibitors could serve as an attractive pharmacological target to decrease thrombotic risk without increasing bleeding. However, the investigators state that the site of VLK action is most likely inside the cell along the secretory route, and not in the extracellular space, although dense granule release should provide sufficient ATP to drive phosphorylation reactions via VLK outside of the cells. Thus, a cell-permeable VLK inhibitor would be necessary, which might also affect other VLK functions and may eventually result in unwanted side effects.
Although the crucial role of VLK in primary hemostasis is clearly demonstrated by Revollo et al, further research is needed to fully explore its precise mode of action (see figure). For example, can VLK also act as serine/threonine kinase? Because the investigators used immune-precipitation with anti-phosphotyrosine antibodies before mass spectrometry–based proteomics, the chance of detecting serine/threonine phosphorylation is reduced. However, the latter may also be catalyzed by VLK based on 2 catalytic domains of the enzyme and some observations that it may also serve as serine/threonine kinase.4 The supplemental data for the article show that ∼60% of identified phospho-sites were actually serine or threonine phosphorylations. The notion that VLK might also act as serine/threonine kinase is also supported by the closeness of the paralog of VLK, TGF-β–activated kinase 1 (MAP3K7), which is a serine/threonine MAPK. Looking at the overlap of phosphorylated putative VLK substrates identified by Whitman and colleagues3 and the proteins being phosphorylated after activation of platelets in the current work reveals 17 proteins. Most of these proteins are phosphorylated on serine or threonine residues, with only 1 being the well-defined extracellular protein (MUC16) and another acting as an endoplasmic reticulum chaperone (HSP90B1). All of the other proteins localize predominantly in the cytosol or the nucleus. These aspects raise the question of whether a fraction of VLK is actually synthesized in the cytosol, potentially as a truncated isoform lacking the signal peptide, either as a result of alternative splicing giving rise to different messenger RNA variants or by the use of alternative start codons. This could also explain an effect of VLK on dense granule release, whereas full-length VLK resides in α-granules.
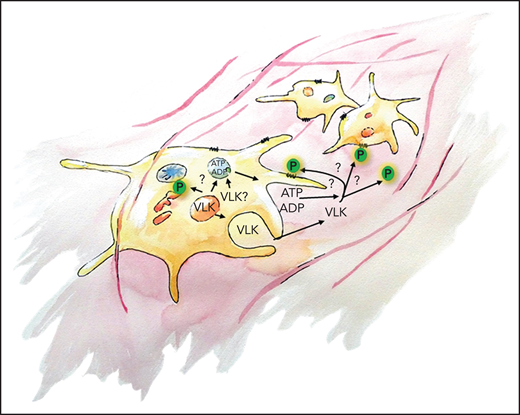
VLK is important for thrombus formation in vivo. VLK is primarily stored in α-granules of platelets and is released upon activation. It is crucial for exocytosis of dense granules; however, the precise mechanism is unknown. VLK also acts as an extracellular kinase and can result in autocrine and paracrine substrate phosphorylation (P). VLK is important for platelet aggregation and supports fibrin deposition.
VLK is important for thrombus formation in vivo. VLK is primarily stored in α-granules of platelets and is released upon activation. It is crucial for exocytosis of dense granules; however, the precise mechanism is unknown. VLK also acts as an extracellular kinase and can result in autocrine and paracrine substrate phosphorylation (P). VLK is important for platelet aggregation and supports fibrin deposition.
Although questions remain, the work of Revollo et al adds important clues to unraveling the multifaceted role of VLK by demonstrating the crucial and complex involvement of this kinase in platelet aggregation and granule secretion.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal