In this issue of Blood, Shen et al1 investigate the role exerted by the transcription factor SOX9 in modulating 24-dehydrocholesterol reductase (DHCR24)-mediated cholesterol synthesis. They report that this pathway is active in a subgroup of aggressive diffuse large B-cell lymphomas (DLBCLs) harboring the IGH-BCL2 translocation, which accounts for ∼10% of DLBCLs.
SOX9 is a member of the SOX family of transcription factors. It plays a critical role in cell fate determination, differentiation, and proliferation, as well as in the maintenance of the stem cell pool in a variety of developing and adult tissues.2 SOX9 overexpression has also been implicated in human cancers, such as hepatocellular carcinoma, breast, bladder, gastric, prostate, pancreatic, and colorectal cancers.3 In this study, Shen et al show that SOX9 is overexpressed preferentially in the IGH-BCL2+ DLBCL, and its expression correlates with advanced stages of disease. To investigate whether SOX9 plays a role in lymphomagenesis, they evaluate SOX9-silenced DLBCL cells and show that SOX9-silenced cells undergo reduced proliferation, G1/S cell-cycle arrest, and apoptotic cell death both in vitro and in vivo. By whole-transcriptome analysis and chromatin immunoprecipitation–sequence assays, the authors identify DHCR24, an enzyme involved in cholesterol biosynthesis, as a direct target of SOX9. Indeed, the enforced expression of DHCR24 is capable of rescuing functions associated with SOX9 knockdown in DLBCL cells by modulating cellular cholesterol levels.
Cholesterol plays a key role in maintaining cell membrane integrity and in regulating cell proliferation of normal, nonmalignant cells.4 It is therefore not surprising that cholesterol homeostasis also participates in the regulation of survival and expansion of leukemic and lymphoma cells, as well as in the protection of these tumor cells from therapy.5 As a consequence, the blockade of cholesterol synthesis induces cell-cycle arrest in different types of leukemia and lymphoma, ultimately leading to apoptotic cell death.6 The identification of the SOX9-DHCR24 axis adds a further layer of complexity to the regulation of cellular cholesterol levels. Indeed, cholesterol homeostasis is mainly regulated by the Sterol Regulatory Element Binding Proteins (SREBPs),7 particularly SREBP2, and by Liver X Receptors (LXRα and β isoforms).8 SREBP2 activates the transcription of enzymes of the mevalonate pathway, such as HMGCR, and regulates the uptake of cholesterol through the induction of low-density lipoprotein receptor expression.4 On the other hand, LXRs control the reverse cholesterol transport pathway, through which the excess cholesterol is returned to the liver for excretion as bile acids.4 In this scenario, the newly identified SOX9-DHCR24 axis highlights a new pathway of cholesterol regulation, which might be particularly relevant for the survival and proliferation of aggressive lymphomas under oncogenic pressure. Of note, the noncanonical regulation of cholesterol synthesis genes by transcription factors in hematopoietic malignancies was reported to occur in multiple myeloma by means of the Interferon Regulatory Factor 4 (IRF4).9 Indeed, IRF4 was found to regulate membrane biogenesis by controlling many enzymes and regulators of sterol and lipid synthesis, such as the squalene epoxidase and the stearoyl-CoA desaturase.9 The results by Shen et al provide compelling evidence of the molecular mechanisms by which SOX9 activates DHCR24 expression and, therefore, cellular cholesterol remodeling. However, the mechanism through which SOX9 is preferentially overexpressed in the IGH-BCL2+ DLBCL remains poorly understood and deserves further investigation.
The results by Shen et al have important therapeutic implications, as they also report the successful treatment of DBLCL-bearing mice with the widely used blood cholesterol-lowering drugs, statins. These drugs, which block the rate-limiting enzyme of cholesterol synthesis (ie, the 3-hydroxy-3methylglutaryl-CoA reductase),7 inhibit DLBCL xenograft tumorigenesis, especially the DLBCL cell lines with higher SOX9 expression (see figure). These results are in accordance with studies demonstrating that the treatment of myeloid leukemia samples with statins enhances chemotherapy-induced leukemic cell apoptosis, by counteracting the cytoprotective increase of the cellular cholesterol levels induced by chemotherapy.6 The synergy between chemotherapy and statins has indeed been explored with encouraging but not definitive results in patients with relapsed acute myeloid leukemia.10 In this context, the study by Shen et al suggests exploring the combination of statins and standard treatments in advanced stage patients affected by SOX9 overexpressing DLBCL.
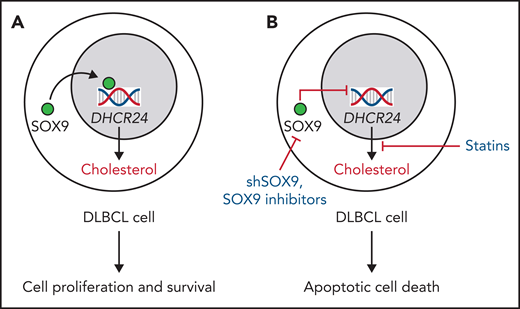
Effects of cholesterol regulation by SOX9-DHCR24 axis in DLBCL. (A) Aggressive DLBCLs harboring the IGH-BCL2 translocation overexpress SOX9, which in turn activates the DHCR24, leading to cholesterol synthesis. Increased cellular cholesterol levels regulate DLBCL cell survival and proliferation. (B) The blockade of SOX9-DHCR24 axis by short hairpin-SOX9 RNA, specific SOX9 inhibitors, or simvastatin inhibits cholesterol synthesis, reduces cellular cholesterol levels, ultimately mediating DLBCL apoptotic cell death both in vitro and in vivo. This axis could be exploited in combination with DLBCL-specific therapy to treat patients affected by advanced forms of DLBCL overexpressing SOX9.
Effects of cholesterol regulation by SOX9-DHCR24 axis in DLBCL. (A) Aggressive DLBCLs harboring the IGH-BCL2 translocation overexpress SOX9, which in turn activates the DHCR24, leading to cholesterol synthesis. Increased cellular cholesterol levels regulate DLBCL cell survival and proliferation. (B) The blockade of SOX9-DHCR24 axis by short hairpin-SOX9 RNA, specific SOX9 inhibitors, or simvastatin inhibits cholesterol synthesis, reduces cellular cholesterol levels, ultimately mediating DLBCL apoptotic cell death both in vitro and in vivo. This axis could be exploited in combination with DLBCL-specific therapy to treat patients affected by advanced forms of DLBCL overexpressing SOX9.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal