Abstract
Introduction:
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare myeloid malignancy characterized by plasmacytoid dendritic cells that aberrantly express interleukin-3 receptor alpha (CD123). It is highly aggressive, associated with a poor prognosis, and the median age at diagnosis is 60-70 years (yr). Clinical presentation is marked by skin and bone marrow involvement; secondary sites include peripheral blood, lymph nodes, and viscera. Tagraxofusp (TAG, SL-401), a first-in-class CD123-targeted therapy, is the only FDA- and EMA-approved treatment for BPDCN. The pivotal trial (NCT02113982) demonstrated that treatment with TAG 12 mcg/kg resulted in a complete response (CR) or clinical CR (CRc; CR with residual skin abnormality not indicative of active disease) in 57% of patient (pts), an overall response rate (ORR) of 75%, and a duration of response (DOR) of 24.9 months (mo) in first-line (1L) pts. Safety profile is well characterized and manageable in 1L and relapsed/refractory (R/R) pts with BPDCN. We report a sub-analysis of this trial stratifying 1L pts by age and baseline site of disease involvement (skin-only, systemic-only, and skin + systemic).
Methods:
We conducted a multicenter, 4-stage, single-arm phase 1/2 trial enrolling 1L (n=69) and R/R (n=20) pts with BPDCN. Pts received TAG IV infusions once daily on days 1-5 of a 21-day cycle. In Stage 1 (dose escalation), TAG was dosed at 7 or 12 mcg/kg; in Stages 2 (expansion), 3 (pivotal, confirmatory), and 4 (continued access), TAG was dosed at 12 mcg/kg. Main outcome measures were CR (defined as CR + CRc), ORR, overall survival, and safety.
Results:
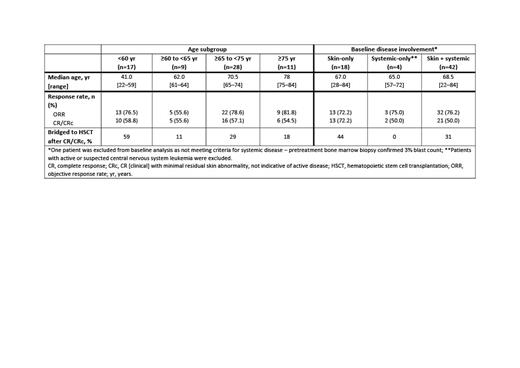
In total, 65 1L pts received TAG at 12 mcg/kg. Most were male, reflective of real-world pts. Age and efficacy outcomes by age and baseline disease involvement are presented in the Table. Similar rates of CR/CRc and ORR were seen across the age cohorts and across the cohorts stratified by baseline disease involvement. In 1L BPDCN pts ≥75 yr (n=11), CR/CRc and ORRs were 55% and 82%, respectively, and similar to pts <60 yr (n=17; CR/CRc and ORRs of 59% and 77%). Pts with skin + systemic baseline disease (65%) had an ORR of 76%, and a CR/CRc rate of 50%. Twenty-one pts bridged to hematopoietic stem cell transplantation (HSCT; median age 63 yr, range 22-78); most (58%) had baseline disease in ≥2 sites, with a probability of survival at 12 and 24 mo of 81% and 69%, respectively. Most HSCTs were observed in pts <60 yr (n=10; 59%) and in pts with baseline skin-only disease (n=8; 44%). In the 44 pts on TAG who were not transplanted (median age 70 yr; range 23-84), 4 pts had prolonged responses (>6 mo, including 2 pts with DOR of 23 and 52 mo); the probability of survival at 12 and 24 mo was 42% and 24%, respectively. Most common treatment-emergent adverse events (TEAEs) across all age cohorts were increased alanine aminotransferase (ALT; <60 yr: 82%; ≥60 to <65 yr: 78%; ≥65 to <75 yr: 59%; ≥75 yr: 55%) and increased aspartate aminotransferase (AST; <60 yr: 71%; ≥60 to <65 yr: n=78%; ≥65 to <75 yr: 52%; ≥75 yr: 55%). Other TEAEs occurring at an incidence >50% were pyrexia, thrombocytopenia, hypoalbuminemia, and fatigue. In pts stratified by baseline disease involvement, the most common TEAEs were ALT and pyrexia (53% each) in the skin-only group; increased ALT, increased AST, fatigue, and nausea (75% each) in the systemic-only group; increased ALT (71%) and increased AST (64%) in the skin + systemic group. In total, 13 pts experienced capillary leak syndrome (CLS; grade [gr] 3, n=3; gr 4, n= 1; gr 5, n=2 ; the highest incidence of CLS occurred in pts aged ≥65 to <75 yr (5 cases; gr 3, n=2; gr 4, n=1) and in pts with skin + systemic disease (7 cases; gr 3, n=1; gr 5, n=1).
Conclusions:
TAG as a first-line treatment for BPDCN was efficacious across all age cohorts including older pts and pts with significant baseline disease. High rates of durable CRs enabled over half of the pts in CR to be bridged to HSCT, including older adults and pts with extensive baseline disease. Some pts who were not transplanted and maintained on TAG had meaningful relapse-free survival - up to 4 yr in one case. Safety events were manageable, transient, mainly occurring in cycle 1, and similar for older pts vs younger pts. Baseline disease involvement did not appear to predispose pts to different TEAEs.
Pemmaraju: ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Bristol-Myers Squibb Co.: Consultancy; LFB Biotechnologies: Consultancy; Aptitude Health: Consultancy; CareDx, Inc.: Consultancy; Affymetrix: Consultancy, Research Funding; Blueprint Medicines: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Clearview Healthcare Partners: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Roche Diagnostics: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Other, Research Funding; Plexxicon: Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; MustangBio: Consultancy, Other; DAVA Oncology: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Springer Science + Business Media: Other; Protagonist Therapeutics, Inc.: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Sager Strong Foundation: Other; Incyte: Consultancy; Celgene Corporation: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy; Samus: Other, Research Funding. Konopleva: AstraZeneca: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Cellectis: Other: grant support; Forty Seven: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Agios: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Ascentage: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Rafael Pharmaceuticals: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; KisoJi: Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding. Sweet: AROG: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Stein: Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Vasu: Seattle Genetics: Other: travel support; Boehringer Ingelheim: Other: Travel support; Kiadis, Inc.: Research Funding; Omeros, Inc.: Membership on an entity's Board of Directors or advisory committees. Rizzieri: Celltron/Teva: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: presentation to FDA for biosimilar review ; Mustang: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Jazz: Other: personal fee; Gilead: Other: personal fee; Incyte: Other: personal fee; Amgen: Other: personal fee; Kite: Other: personal fee; AROG: Other; Pharmacyclics: Other. Wang: AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rafael Pharmaceuticals: Other: Data safety monitoring committee; DAVA Oncology: Consultancy, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory board; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Other: Advisory Board; Mana Therapeutics: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy. Kantarjian: Ipsen Pharmaceuticals: Honoraria; AbbVie: Honoraria, Research Funding; Jazz: Research Funding; Astellas Health: Honoraria; Pfizer: Honoraria, Research Funding; Aptitude Health: Honoraria; Precision Biosciences: Honoraria; Astra Zeneca: Honoraria; Taiho Pharmaceutical Canada: Honoraria; KAHR Medical Ltd: Honoraria; NOVA Research: Honoraria; Daiichi-Sankyo: Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Research Funding; BMS: Research Funding; Novartis: Honoraria, Research Funding; Immunogen: Research Funding. Brooks: Stemline Therapeutics: Current Employment. Mughal: Stemline: Current Employment, Current holder of stock options in a privately-held company; Oxford University Press, Informa: Other: financial benefit and/or patents . Lane: Qiagen: Consultancy, Honoraria; N-of-One: Consultancy, Honoraria; Stemline Therapeutics: Research Funding; AbbVie: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal