Abstract
Background
Sickle cell disease (SCD) is an autosomal recessive disorder where mutated hemoglobin (HbS) polymerizes and can lead to irreversible red blood cell (RBC) sickling and painful vaso-occlusive crisis (VOC). The RBC sickling is amplified by inflammation, resulting in tissue and organ damage. The transcription factor Nuclear factor erythroid 2-related factor 2 (Nrf2) coordinates the expression of antioxidant genes in response to oxidative stress, regulates inflammation, inhibits the NFkB pathway, and induces fetal hemoglobin (HbF), making it an attractive target in SCD and beta-thalassemia. IMR-261 is a novel oral activator of Nrf2 and has been tested in Phase 2 clinical trials (previously as CXA-10).
Methods & Results
CD14+ human monocytes were exposed to IMR-261 at 3µM and 10µM for 3 hours, to determine via quantitative PCR (qPCR) its ability to induce expression of antioxidant genes. IMR-261 at 10 µM significantly increased (p<0.05) the expression of Nrf2-dependent genes (p<0.05), including HMOX1, HSPA1A, HSP90, GCLM, SOD1 and TXNRD1. Human monocytes were treated with lipopolysaccharide (LPS) to test the ability of IMR-261 to block inflammatory genes with a NFkB target dataset. IMR-261 significantly inhibited (p<0.05) LPS-induced expression of IL-1-beta, TNF-alpha and IL-6 in human monocytes.
To test the effects of IMR-261 on HbF induction, human erythroblasts were derived from CD34+ blood marrow progenitor cells sourced from healthy or SCD subjects. IMR-261 induced expression of the gamma-globin gene (4.0-fold change at 3µM and 7.18-fold change at 6 µM). This was accompanied by increased %F-cells (2.8-fold change at 3µM and 3.0-fold change at 6 µM).
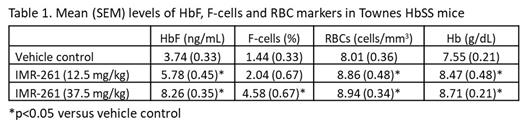
IMR-261 was also tested in the Townes HbSS mouse model of SCD to assess the potential for HbF induction. Mice were dosed with IMR-261 at 12.5 mg/kg or 37.5 mg/kg BID for 4 weeks (N=4-8/group). After 4 weeks of treatment, IMR-261 at 12.5 mg/kg and 37.5 mg/kg resulted in a significant increase in HbF relative to control, and 37.5 mg/kg resulted in a significant increase in %F-cells relative to control (Table 1, p<0.05). In addition, both doses of IMR-261 led to significant increases in RBC counts and total hemoglobin (Hb) (Table 1, p<0.05). IMR-261 at 37.5 mg/kg also significantly decreased (p<0.05) both reticulocyte counts and spleen cellularity.
The ability of IMR-261 to reduce VOCs was assessed in separate Townes HbSS mice after the administration of TNF-alpha (0.5 µg/mice i.p.). IMR-261 was dosed at 37.5 mg/kg BID for 5 days before triggering VOCs. RBCs were stained with Ter-119 antibodies on spleen and liver of mice. Compared to controls, IMR-261 significantly reduced the presence of RBC on occluded vessels. This was coupled with a reduction of P-selectin (3109±97 Mean Fluorescence Units [MFI] in vehicle-treated vs. 1974±379 MFI in IMR-261 group, p<0.05) and L-selectin (375±20 MFI in vehicle-treated vs. 242±60 MFI in IMR-261 group, p<0.05). IMR-261 also reduced select hemolysis biomarkers: bilirubin (11.2±0.3 mg/dL in vehicle-treated vs. 8.4±0.7 mg/dL in IMR-261 group, (p<0.05) and free-heme (325±52 µM in vehicle-treated vs. 203±51 µM in IMR-261 group, p<0.05).
A beta-thalassemia experimental model Hbb th1/th1 was tested to evaluate whether IMR-261 could improve ineffective erythropoiesis seen in beta-thalassemia. IMR-261 treatment at 37.5 mg/kg BID significantly increased hemoglobin levels, RBC counts and hematocrit (p<0.05), with significant reductions observed in reticulocytes (p<0.05). flow cytometry analysis (CD71/Ter119) showed that IMR-261 significantly decreased late basophilic and polychromatic erythroblasts (Ery.B) and increased orthochromatic erythroblasts and reticulocytes (Ery.C) cell numbers in the spleen (p<0.05).
Conclusions
IMR-261 activates Nrf2-dependent antioxidant genes and inhibits NFkB-induced pro-inflammatory genes in human monocytes. In human erythroblasts, IMR-261 significantly increased HbF and %F-cells. In vivo SCD models show that IMR-261 significantly induced HbF and %F-cells, improved hemolytic markers, and decreased VOCs. IMR-261 also increased Hb and improved ineffective erythropoiesis in a beta-thalassemia in-vivo model. Together these data suggest that IMR-261 is a promising, novel, oral therapy that warrants clinical testing in SCD and beta-thalassemia.
Maciel: Imara Inc.: Research Funding. Carvalho: Imara Inc.: Research Funding. Rignault: Imara Inc.: Research Funding. Pace: Imara Inc.: Consultancy. OCain: Imara Inc.: Current Employment, Current equity holder in publicly-traded company. Ballal: Imara Inc.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal