Abstract
Background: Chronic myelomonocytic leukemia (CMML) is an aging disease that arises on the background of clonal hematopoiesis, commonly involving multiple TET2 mutations or TET2/SRSF2 co-mutations. In a prior study of 1084 CMML patients (Leukemia 2020; 34:1407-1421), we demonstrated a high prevalence of TET2 mutations (56%) in CMML, with approximately 30% of patients having >1 TET2 mutation. The cell-type and allele specific distribution of these mutations remains of great interest and are thus far poorly defined. To visualize the genotypic and immunophenotypic correlates, we performed single-cell DNA analysis and simultaneous protein profiling on 2 CMML patients with multiple TET2 mutations.
Methods: After Mayo Clinic IRB approval, two CMML patients with dual TET2 mutations and available peripheral blood mononuclear cells were included in the study. Patient samples were labeled with TotalSeq TM -D Heme Oncology Cocktail (BioLegend, San Diego, CA) consisting of 45 unique oligo-conjugated cell surface markers. The samples were then washed and loaded into a Tapestri microfluidics cartridge, where single cells (3,500 cells/µL) were encapsulated, lysed and barcoded using the MissionBio Myeloid custom amplicon panel consisting of 312 amplicons in 45 genes relevant to myeloid disorders (MissionBio, San Francisco, CA). The individual barcoded DNA and protein for each cell was purified, recovered, and labeled with Illumina i5/i7 indices, quanitifed by Agilent Bioanalyzer and pooled for sequencing. The libraries were sequenced via Illumina NovaSeq by the Mayo Genomics Core, and data was recovered and analyzed using MissionBio Tapestri Insights v3.0.2.
Results: Two dual TET2 mutated CMML patients were included in the study, both male, ages 79 and 83, and both with CMML-0. Case 1 had TET2 p.G1172Efs*53 (variant allele fraction/VAF-6%) and p.H1732Tfs*13 (VAF-13%) mutations, while case 2 had TET2 p.Y620Ffs* (VAF-85%), p.G898* (VAF-82%), and SRSF2 p.95H (VAF-45%) mutations, respectively. In both patients, TET2 mutations were found to be preferentially distributed in monocytes (CD11b+, CD14+, CD64+, CD13+, CD33+, HLA-DR+), with very few classically defined granulocyte (CD16+,CD13+.CD33+, HLA-DR neg), B (CD19+,CD22+)/T (CD2+,CD4+,CD5+,CD7+) lymphocytes, NK cells (CD16+/CD56+/HLA-DR neg) and plasma cells (CD38+/CD138+), demonstrating mutant TET2. Among monocytes, TET2 mutations were enriched in classical/M01 (CD11b+, CD14+,CD16-) and intermediate/M02 monocytes and were infrequent in the MO3 fraction, commensurate with monocyte repartitioning seen in CMML. Interestingly, a large number of single cells with TET2 mutations also demonstrated an aberrant surface phenotype, with universal expression of CD14+ and CD11b+, along with a mixture of other markers (CD163+, CD11c, CD4+, CD2+, and/or CD56+). With regards to allelic distribution, while several permutations and combination were seen involving heterozygosity and homozygosity for the TET2 mutations identified on bulk sequencing, data was corroborative for both TET2 mutations being located on trans alleles.
Conclusions: In this study, we demonstrate that TET2 mutations in CMML are highly enriched in classical circulating monocytes and infrequently occur in classical granulocytes, B/T and NK cells; with multiple TET2 mutations most commonly co-existing on trans alleles. Analyses on additional samples along with copy number variation assessments are ongoing.
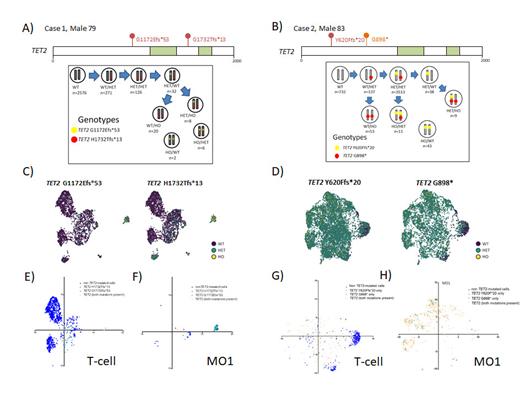
Figure 1: Lollipop plot of TET2 exons with dual TET2 mutations labeled above and oxygenase domain labeled in green; below are number of cells identified with each genotype for Case 1 (A) and Case 2 (B). Mutations are labeled proximal (yellow) and distal (red) and alleles are in grey. The directionality of the arrows to different genotypes is based on decreasing cell number and genotype. Uniform manifold approximation and projection (UMAP) plot of Case 1 (C) and Case 2 (D) with cells clustered by immunophenotype and labeled by genotype (non TET-2 mutated purple, heterozygous TET2 in green, homozygous TET2 in yellow). Relative protein expression of T-cell phenotype (CD14-, CD3+, CD4+/-,CD8+/-, CD5+, CD7+, CD2+), or classical monocytes (CD14+/CD16-) for Case 1 (E & F) and Case 2 (G & H) overlayed on UMAP. Royal blue represents non TET2-mutated cells, and mutated cells are labeled in pastel blue, green and light orange by mutation type.
Durruthy-Durruthy: Mission Bio Inc.: Current Employment. Arribas-Layton: Mission Bio, Inc.: Current Employment. Patnaik: StemLine: Research Funding; Kura Oncology: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal