TO THE EDITOR:
Current blood group antibody detection technologies use red blood cells (RBCs) as antigen matrix and detection tool. Anti-CD38 and anti-CD47 are the first of several emerging medicinal monoclonal antibodies (mAbs) targeting broadly expressed antigens that are also found on RBCs. Thus, the medicinal antibodies pan-agglutinate the test RBCs and interfere with antibody screening and identification in indirect antiglobulin tests (IATs). So far, only anti-CD38 mAbs (daratumumab [DARA],1 isatuximab2 ), licensed for multiple myeloma, are routinely available in clinical practice. Because multiple myeloma patients frequently have transfusion needs,3 the artificial pan-agglutination in IAT is a relevant clinical problem. With rare exceptions, most currently used antibody screening and identification technologies are sensitive to this artifact. To circumvent this issue, laboratories incubate test RBCs with dithiothreitol (DTT),4 which destroys CD38 as well as several other transfusion-relevant polymorphic blood group antigens, most notably KEL1 (K) and KEL2 (k) and other antigens of the Kell blood group system.5 Therefore, transfusion is based on pre-emptive matching for the relevant KEL antigens, whereas the small risk of transfusing against antibodies against other DTT-sensitive antigens (DO, JMH, LU, IN, or YT) is accepted. However, extended pheno- or genotyping of the patient is recommended to reduce the immunization risk.4,6,7 This strategy also simplifies identification of compatible blood products and can reduce the number of antibody screening tests.8-10 Moreover, treatment of test RBCs with DTT puts the test cells outside of their validated specification and downgrades the registration status of the IAT to in-house. Lastly, DTT will not be useful for other upcoming ubiquitous antigens targeted with immunotherapies, such as CD47 (magrolimab), which is a promising target for a wide spectrum of cancers.11
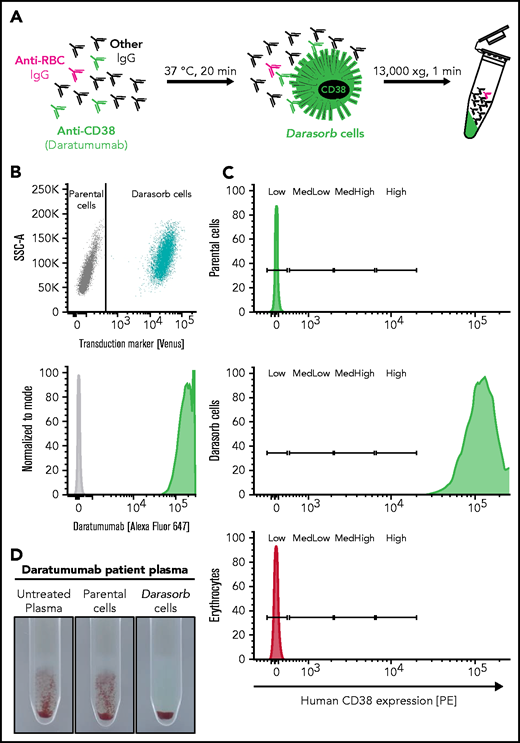
We generated and validated an innovative reagent to facilitate immunohematology testing in anti-CD38–treated patients, namely cells for preanalytic absorption of free anti-CD38 from diagnostic patient plasma, which we call “Darasorb.” Darasorb cells are incubated with analytic patient plasma and subsequently removed by centrifugation. DARA depletion is achieved by incubation of 75 µL of plasma with 3 × 107 Darasorb cells for 20 minutes, at 37 °C, with 950 rpm shaking in a Thermomixer R (Eppendorf, Hamburg, Germany). Thereafter, Darasorb cells are pelleted by centrifugation for 1 minute in a MIKRO 120 tabletop centrifuge (Hettich, Tuttlingen, Germany) at full speed. Afterward anti–CD38-depleted plasma is subjected to immunohematology testing as per the manufacturer’s instructions. The test principle can easily be adapted to other antigens. A schematic overview is shown in Figure 1A. Briefly, Darasorb cells were generated by stable transduction of MEL-745A cells (German Collection of Microorganisms and Cell Cultures; #ACC 501) with a human CD38 expression cassette (#HG10818-M; Sino Biological, Wayne, PA) and subcloning of high expressers based on fluorescence-labeled daratumumab binding (Figure 1B). Expression under a strong promoter facilitates very high surface expression, with ∼5000-fold more CD38 molecules per cell than on RBCs (Figure 1C). Low, but immunohematologically relevant, CD38 expression on RBCs has been reported previously.12 The advantage of xenogeneic cells over latex beads is the presentation of antigens in their natural conformation.13 The choice of cells is likely important. On the one hand, a basic cellular backbone is required for the expression of some of the human membrane proteins in murine cells. On the other hand, serological cross-reactivity with homologous murine proteins would limit the usefulness of our technology. As shown by specific in silico and experimental analyses, despite the similarity of murine and human erythroid protein expression patterns and the structural similarity of murine and human erythroid surface markers,14,15 the concordance, especially around the polymorphic sites, is so small that there is no serological cross-reactivity between MEL-745A cells and human RBCs (supplemental Figure 1, available on the Blood Web site). Detailed proteomics analyses of the MEL-745A cells used here16 confirm their lack of potentially cross-species reactive antigens; indeed, parental control cells did not interfere with the irregular antibodies (Figure 1D). Manufacturing and quality control are simple, and the generation of absorption cells for other antibody specificities is rapid. Cells can be stored at 4 °C in TransFix fixative solution (Cytomark, Little Balmer, United Kingdom) for ≥14 days (supplemental Figure 2).
Schematic overview and characterization of Darasorb cells. (A) The schematic diagram shows a pan-agglutinating plasma of a patient treated with anti-CD38 (eg, DARA). Detection of irregular antibodies (anti-RBCs) within the plasma is obscured by pan-agglutination due to anti-CD38 mAbs. Nonhuman cells expressing human CD38 at high density (here called “Darasorb” cells) deplete free medicinal antibody from patient plasma, whereas other antibodies remain unaffected. Subsequently, Darasorb cells are removed by centrifugation, whereas all other antibody species are preserved. (B) Nonhuman cells were stably transduced to coexpress human CD38 antigen and the fluorochrome Venus. CD38-expressing Darasorb cells bind DARA (labeled with Alexa Fluor 647). (C) Approximate antigen density determination was achieved with BD Quantibrite beads bearing a defined number of phycoerythrin (PE) molecules on their surface as reference. The PE molecules per cell are divided into “low,” “medium low,” “medium high,” and “high.” Cells of interest were analyzed for human CD38 surface expression with an anti-human CD38-PE antibody. Murine parental cells, Darasorb cells, and human erythrocytes are shown. A Darasorb cell displays ∼490 000 copies of CD38 compared with human erythrocytes, with ∼100 CD38 molecules per RBC. (D) Reactivity of a plasma sample from a patient treated with DARA is shown in the gel agglutination test. The untreated plasma (left panel) shows the expected agglutination in the IAT. The reaction is not sensitive to incubation with parental cells (middle panel). The same plasma treated with Darasorb cells (right panel) is devoid of reactivity, confirming quantitative depletion of DARA.
Schematic overview and characterization of Darasorb cells. (A) The schematic diagram shows a pan-agglutinating plasma of a patient treated with anti-CD38 (eg, DARA). Detection of irregular antibodies (anti-RBCs) within the plasma is obscured by pan-agglutination due to anti-CD38 mAbs. Nonhuman cells expressing human CD38 at high density (here called “Darasorb” cells) deplete free medicinal antibody from patient plasma, whereas other antibodies remain unaffected. Subsequently, Darasorb cells are removed by centrifugation, whereas all other antibody species are preserved. (B) Nonhuman cells were stably transduced to coexpress human CD38 antigen and the fluorochrome Venus. CD38-expressing Darasorb cells bind DARA (labeled with Alexa Fluor 647). (C) Approximate antigen density determination was achieved with BD Quantibrite beads bearing a defined number of phycoerythrin (PE) molecules on their surface as reference. The PE molecules per cell are divided into “low,” “medium low,” “medium high,” and “high.” Cells of interest were analyzed for human CD38 surface expression with an anti-human CD38-PE antibody. Murine parental cells, Darasorb cells, and human erythrocytes are shown. A Darasorb cell displays ∼490 000 copies of CD38 compared with human erythrocytes, with ∼100 CD38 molecules per RBC. (D) Reactivity of a plasma sample from a patient treated with DARA is shown in the gel agglutination test. The untreated plasma (left panel) shows the expected agglutination in the IAT. The reaction is not sensitive to incubation with parental cells (middle panel). The same plasma treated with Darasorb cells (right panel) is devoid of reactivity, confirming quantitative depletion of DARA.
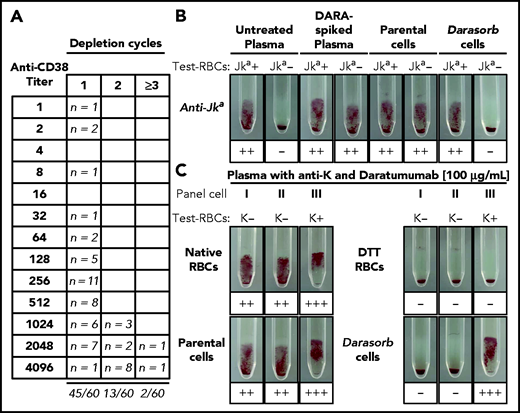
Titration experiments, using 60 plasma samples from 27 patients who were recently treated with daratumumab or isatuximab, were performed. We identified peak plasma titers of 1024 to 4096, although more than half had titers ≤512 (Figure 2A; see supplemental Methods for details). Seventy-five percent of plasma samples were reproducibly cleared of free pan-agglutinin with a single incubation with Darasorb cells; an additional 22% required a second round of depletion. All samples with titers up to 512 were reliably purged with 1 depletion. The need for a second depletion cycle was restricted to samples with high titers ≥ 1024. In only 2 samples with titers of 2048 and 4096, 4 and 3 depletion cycles with Darasorb cells, respectively, were necessary to completely deplete the medicinal antibody. Although attributability of the residual pan-agglutination to an irregular antibody with reactivity against a high-frequency DTT-sensitive antigen could explain the observation, more likely it is simply due to excessive DARA titers (supplemental Figure 3).
Depletion of anti-CD38 from patient plasma using Darasorb cells. (A) Sixty routine plasma samples from 27 patients treated with anti-CD38, 26 patients treated with DARA, and 1 patient treated with isatuximab, were processed with Darasorb cells and subsequently analyzed in gel test cards. Fifty of 60 (97%) investigated samples were anti-CD38 depleted with 1 (n = 45) or 2 (n = 13) depletion cycles with Darasorb cells. Only 2 tested plasma samples remained pan-agglutinating after 2 incubations. (B) Depletion with Darasorb cells quantitatively retains irregular antibodies. Untreated patient plasma containing low-titer anti-Jka antibodies was spiked with DARA, resulting in agglutination with Jka positive (+) and Jka negative (−) RBCs. After treatment with Darasorb cells, only agglutination with the Jka positive (+) RBCs is observed, indicating complete and specific removal of free anti-CD38. The negative control (incubation with parental cells) remained pan-reactive, as expected. (C) IAT with plasma containing DARA and anti-K. A patient plasma containing anti-K antibodies was supplemented with DARA, resulting in pan-agglutination with native test RBCs I, II and III. After treatment of the RBCs with DTT, all reactions are negative, because CD38 and KEL glycoprotein on the test cells are destroyed. After selective depletion of DARA with Darasorb cells, a positive reaction is observed with the KEL1-expressing (K+) test RBCs III. The negative depletion control (parental cells) showed the expected result.
Depletion of anti-CD38 from patient plasma using Darasorb cells. (A) Sixty routine plasma samples from 27 patients treated with anti-CD38, 26 patients treated with DARA, and 1 patient treated with isatuximab, were processed with Darasorb cells and subsequently analyzed in gel test cards. Fifty of 60 (97%) investigated samples were anti-CD38 depleted with 1 (n = 45) or 2 (n = 13) depletion cycles with Darasorb cells. Only 2 tested plasma samples remained pan-agglutinating after 2 incubations. (B) Depletion with Darasorb cells quantitatively retains irregular antibodies. Untreated patient plasma containing low-titer anti-Jka antibodies was spiked with DARA, resulting in agglutination with Jka positive (+) and Jka negative (−) RBCs. After treatment with Darasorb cells, only agglutination with the Jka positive (+) RBCs is observed, indicating complete and specific removal of free anti-CD38. The negative control (incubation with parental cells) remained pan-reactive, as expected. (C) IAT with plasma containing DARA and anti-K. A patient plasma containing anti-K antibodies was supplemented with DARA, resulting in pan-agglutination with native test RBCs I, II and III. After treatment of the RBCs with DTT, all reactions are negative, because CD38 and KEL glycoprotein on the test cells are destroyed. After selective depletion of DARA with Darasorb cells, a positive reaction is observed with the KEL1-expressing (K+) test RBCs III. The negative depletion control (parental cells) showed the expected result.
Because none of the anti-CD38 mAb–treated patients in the care of our immunohematology laboratory had irregular antibodies, we spiked patient plasma with irregular antibodies of various specificities and strengths with DARA. Spiking caused the expected pan-agglutination, as exemplified by a plasma also containing a low-titer anti-Jka antibody (Figure 2B; see supplemental Figure 1 for other specificities). After purging the plasma of free DARA with Darasorb cells, irregular antibodies became clearly detectable with antigen-positive cells; antigen-negative cells were nonreactive as expected. It should be considered that repeated cycles of absorption can potentially dilute and, thus, obscure very weak alloantibodies. Importantly, antibody detection was equally sensitive in untreated and spiked depleted plasma; in most cases, even very weak antibodies were quantitatively retrieved after plasma purging. A salient advantage of Darasorb cells over DTT pretreatment of test RBCs is their ability to facilitate detection of antibodies to antigens in the KEL blood group system. While DTT cleaved the KEL proteins so that a plasma’s anti-K reactivity remained undetected, Darasorb selectively removed DARA, whereas anti-K was sensitively detected with K-heterozygous test RBCs (Figure 2C).
As an alternative approach, neutralization of free DARA by addition of soluble antigen has been proposed.4 Although this is a potentially relevant competitor, we identify certain advantages of our technology. Technically, generation and purification of recombinant membrane proteins are more difficult than their expression in cells; their stabilization in a conformation recognized by a given antibody may not be without complexity. Moreover, unless the neutralizing reagent is delivered as a dry powder, sample dilution by the reagent can obscure weakly reactive antibodies.
In summary, immunohematology work-up of any patient intended to receive anti-CD38 mAbs entails extended immunophenotyping, and all patients in need of transfusions must receive K/k-matched RBCs. A reagent like Darasorb could remove these requirements. As we are showing for a panel of clinically relevant irregular antibodies, Darasorb reagent clears plasma of DARA without interfering with the detection of weak irregular antibodies. Darasorb cells and, by extension, absorption cells for other medicinal antibodies, could be useful additions to the immunohematological repertoire. Absorber cells for antibodies against frequent or high-frequency antigens (especially Vel) could be similarly generated and facilitate the detection and specification of further irregular antibodies.
Acknowledgments
The authors thank the immunohematology technicians for providing patient plasma and performing blinded IAT.
A portion of this work was performed in the framework of the BSc thesis of P.M.
Authorship
Contribution: E.E. conceived, performed, and supervised experiments, analyzed data, and generated figures; P.M. and S.H. performed experiments and analyzed data; C.G. conceived and supervised experiments; H.B. conceived and supervised experiments, analyzed data, and wrote the manuscript; and E.S. and H.B. share the overall responsibility for the work. All authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.G. and H.B. invented the technology that is the basis of this study and is registered world-wide (EP3062109A1/JP2017545404A/CA2977436A/US15/552 657/PCT/EP2016/053989/AU2016223431A). E.S. is the Chief Medical Officer of the company that owns the intellectual property according to the German Law on Employees' Inventions. The remaining authors declare no competing financial interests.
Correspondence: Halvard Bonig, Goethe University School of Medicine, Haus 76, Sandhofstraße 1, 60528 Frankfurt am Main, Germany; e-mail: h.boenig@blutspende.de, hbonig@uw.edu.
Presented in abstract form by E.E. at the 6 March 2020 Interdisciplinary Group for Laboratory Diagnostics and Flow Cytometry (IGLD) meeting in Frankfurt, Germany and the digital German Society for Transfusion Medicine and Immunohematology (DGTI) meeting 2020.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal