Abstract
The introduction of targeted immunotherapies specifically, brentuximab vedotin (BV) and programmed death-1 (PD-1)–blocking antibodies (nivolumab and pembrolizumab), has reshaped the therapeutic landscape of classical Hodgkin lymphoma (cHL) in the past decade. Targeting specific biologic features of cHL, these novel agents have expanded treatment options for patients with multiply R/R cHL and have increasingly been studied at earlier points in a patient’s disease course. With the plethora of studies evaluating BV and PD-1 blockade as part of cHL therapy, often in nonrandomized, controlled studies, more questions than answers have arisen about how to optimally integrate these drugs into clinical practice. In this article, we use a case-based format to offer practical guidance on how we incorporate BV and anti-PD-1 antibodies into the management of cHL and review the data supporting those recommendations.
Introduction
Classical Hodgkin lymphoma (cHL) is curable in most patients, but 10% to 30% of patients who receive standard therapy will develop relapsed or refractory (R/R) cHL.1-5 Although high-dose chemotherapy and autologous hematopoietic cell transplantation (auto-HCT) is the standard treatment of chemotherapy-sensitive patients with R/R cHL, only about half of patients will attain durable disease control.6-10
The advent of novel treatment options for cHL has revolutionized the cHL therapeutic landscape. Brentuximab vedotin (BV) is an antibody-drug conjugate with a microtubule toxin payload (monomethyl auristatin E) directed against CD30 on the surface of Hodgkin Reed-Sternberg cells that has been approved by the US Food and Drug Administration (USFDA) for the treatment of advanced-stage and R/R cHL.11 Also approved for use in R/R cHL by the USFDA, the programmed death-1 (PD-1)–blocking antibodies nivolumab and pembrolizumab interrupt the interaction between PD-1 and its ligands PD-L1 and -L2, which are overexpressed in cHL because of the aberrations in chromosome 9p24.1 (containing the PD-L1 and PD-L2 loci) and play a fundamental role in the pathogenesis of the disease.12 These immunotherapies targeting specific biologic features of cHL have expanded the therapeutic armamentarium for patients with multiply R/R cHL and have now been studied in various settings throughout a patient’s disease course, from initial therapy to posttransplant consolidation. With the abundance of studies that have evaluated BV and PD-1 blockade as part of cHL therapy, often in nonrandomized, controlled studies, the dilemma of how to optimally integrate these drugs into clinical practice has arisen. In this article, we use a case-based format to offer practical guidance on how to incorporate BV and anti-PD-1 antibodies into the management of cHL.
Case 1
A 24-year-old man with stage III cHL was treated with 2 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by 4 cycles of AVD after a post–cycle 2 positron emission tomography-computed tomography (PET-CT) demonstrated a complete metabolic response (CMR).4 An end of treatment (EOT) PET-CT confirmed CMR. Fourteen months after treatment, he noted right cervical node enlargement. Restaging PET-CT showed hypermetabolic adenopathy (cervical, mediastinal, and bilateral axillary nodes), and a lymph node biopsy specimen showed Reed-Sternberg cells with bilobed nuclei that were positive for CD15, CD30, and dim PAX5 and negative for CD45 and CD3, among abundant nonmalignant inflammatory cells. He received 2 cycles of ifosfamide, carboplatin, and etoposide (ICE) with CMR and subsequent auto-HCT. Six months later, he felt a palpable right axillary node, and restaging PET-CT showed hypermetabolic bilateral axillary and mediastinal lymph nodes. Biopsy results confirmed relapsed cHL.
BV and PD-1 blockade in multiply R/R cHL
A pivotal phase 2 study in patients (n = 102) with R/R cHL after auto-HCT demonstrated that BV (1.8 mg/kg every 3 weeks, maximum 16 cycles) produced an (ORR) of 75% and complete response (CR) rate of 34%.13 Among the patients with CR, responses were durable, with a 5-year overall survival (OS) and progression-free survival (PFS) of 64% and 52%, respectively.14 The most common adverse event (AE) was peripheral neuropathy (56%).13
PD-1 blockade is also effective in patients who progress after auto-HCT. In the pivotal phase 2 CheckMate 205 study, nivolumab (3 mg/kg every 2 weeks) produced an ORR of 69% and CR rate of 16% in R/R patients with cHL in whom prior auto-HCT and/or prior BV had failed.15,16 Pembrolizumab (200 mg every 3 weeks) had similar efficacy in a pivotal phase 2 study of patients with R/R cHL in whom auto-HCT had failed or in patients refractory to salvage therapy and BV with an ORR of 72% and CR rate of 28%.17,18 Both anti-PD-1 antibodies produced similar response rates across patient subgroups, regardless of the number of prior lines of therapy or receipt of prior auto-HCT or BV. In both studies, response duration was associated with depth of response to anti-PD-1 monotherapy (eg, CM205: median duration of response 20.3 months after CR, 12.8 months after partial response.18
Anti-PD-1 monotherapy is well tolerated but is associated with immune-related AEs (irAEs) that result in therapy discontinuation in 5% to 7% of patients. Most irAEs are grade 1/2, including hypothyroidism and hyperthyroidism (12% to 16%), rash (9%), hepatitis (5%), and pneumonitis (3% to 4%).16,17 Anti-PD-1 therapy can be continued if asymptomatic endocrinopathy occurs, although it should be discontinued in the presence of severe irAEs, which are managed with corticosteroids, with or without other immunosuppressive therapies. Rechallenge with PD-1 blockade after irAE resolution and corticosteroid taper can be considered in certain circumstances.19,20
How do we incorporate novel agents for patients with multiply R/R cHL?
Novel agent naïve multiply R/R cHL including post-autoHCT relapse
Although both BV and PD-1 inhibitors are effective in multiply R/R cHL, there is no consensus on the optimal timing and sequencing of these drugs. The phase 3 randomized KEYNOTE-204 study comparing pembrolizumab 200 mg (n = 148) to BV 1.8 mg/kg (n = 152) every 3 weeks for up to 35 cycles in patients with R/R cHL21 showed that pembrolizumab was associated with significantly longer PFS compared with BV (hazards ratio [HR], 0.65; 95% confidence interval, 0.48-0.88; median, 13.2 vs 8.3 months). The benefit was noted across subgroups, including primary refractory cHL (HR, 0.52).21 Pembrolizumab and BV were associated with a similar incidence of grade ≥3 AEs (19.6% vs 25%), but pembrolizumab was associated with a higher incidence of irAEs (hypothyroidism [15.5% vs 1.3%] and pneumonitis [10.8% vs 2.6%]), whereas BV was associated with more nausea (13.2% vs 4.1%) and peripheral neuropathy (18.4% vs 2%). In case 1, the patient received chemotherapy-based initial and salvage therapy, did not receive consolidation after auto-HCT, and was thus naïve to BV and PD-1 blockade. In patients with multiply R/R cHL who have not received either agent, we recommend using PD-1 blockade before BV, based on the preliminary results from KEYNOTE-204, although publication of the final analysis is awaited to confirm this recommendation.
Based on available evidence, there is no clear difference in the efficacy or toxicity between pembrolizumab and nivolumab. They are administered on different schedules (every 2 or 4 weeks for nivolumab, every 3 or 6 weeks with pembrolizumab), and the duration of therapy was different in clinical trials (until PD with nivolumab, until PD for a maximum of 2 years, with the option to discontinue early after CR with pembrolizumab). We typically use a personalized approach to antibody and schedule selection, tailored to the patient’s wishes, clinical status, and depth of response. For patients who need closer monitoring, we choose a schedule of nivolumab every 2 weeks or pembrolizumab every 3 weeks. In patients who achieve CR, we treat until progression, with consideration of discontinuation after 6 months (at the earliest) and usually discontinue after 2 years. In preliminary reports, retreatment with PD-1 blockade after discontinuation is associated with an ORR ranging from 67% to 100%22-25 ; therefore, this approach may be logical for patients who wish to pause therapy, although results are unpublished and include a small number of patients. For patients with PR or stable disease in response to PD-1 blockade, we usually treat until progression. In CM205, patients treated beyond progression had a longer median time between PD and next systemic therapy (8.8 months vs 1.5 months) and a longer median OS from the time of PD (not reached vs 13.2 months),16 so we continue treatment beyond progression if the patient is clinically well and the disease burden is not high. Follow-up imaging is critical to distinguish between “pseudoprogression” and true PD.26 In patients with multiply R/R cHL who have progressed on anti-PD-1 therapy, we recommend BV for up to 16 cycles, depending on tolerance of AEs (ie, peripheral neuropathy).
Multiply R/R cHL including post–auto-HCT relapse with prior exposure to novel agents
For patients who have multiply R/R cHL or relapse after auto-HCT with prior BV or anti-PD-1 exposure, we recommend selecting the agent that has not been used previously. Although the ORR to BV or anti-PD-1 monotherapy is high, the CR rate and ultimate curative potential with either type of agent is relatively low.14,16,27 To improve the depth and duration of response, combination strategies are currently being evaluated in clinical trials with promising results thus far, although these approaches are not standard and are not recommended in routine practice at this time (Table 1).28-33
Studies of BV and PD-1 blockade in multiply relapsed/refractory cHL
| Regimen . | Phase . | N . | ORR (%) . | CRR (%) . | NCT no. . | Reference . |
|---|---|---|---|---|---|---|
| BV monotherapy and combination therapy in R/R cHL | ||||||
| BV | 2 | 102 | 75 | 34 | NCT00848926 | 13 |
| BV-ibrutinib | 2 | 39 | 69 | 46 | NCT02744612 | 28 |
| BV-bendamustine | 1/2 | 64 | 71 | 32 | NCT01657331 | 29 |
| BV-ipilimumab | 1 | 21 | 76 | 57 | NCT01896999 | 30 |
| BV-nivolumab | 1 | 18 | 88 | 61 | NCT01896999 | 30 |
| BV-nivolumab-ipilimumab | 1 | 22 | 82 | 73 | NCT01896999 | 30 |
| Anti PD-1 monotherapy and combination therapy in R/R cHL | ||||||
| Nivolumab | 2 | 243 | 69 | 16 | NCT02181738 | 16 |
| Pembrolizumab | 2 | 210 | 69 | 22 | NCT02453594 | 18 |
| Nivolumab+ipilimumab | 1b | 31 | 74 | 23 | NCT01592370 | 31 |
| Pembrolizumab+vorinostat | 1 | 9* | 100 | 44 | NCT03150329 | 32 |
| Camrelizumab+decitabine | 2 | NCT03250962 | 33 | |||
| Anti-PD-1 naive | — | 42 | 95 | 71 | — | |
| Prior anti-PD-1 | — | 25 | 52 | 28 | — |
| Regimen . | Phase . | N . | ORR (%) . | CRR (%) . | NCT no. . | Reference . |
|---|---|---|---|---|---|---|
| BV monotherapy and combination therapy in R/R cHL | ||||||
| BV | 2 | 102 | 75 | 34 | NCT00848926 | 13 |
| BV-ibrutinib | 2 | 39 | 69 | 46 | NCT02744612 | 28 |
| BV-bendamustine | 1/2 | 64 | 71 | 32 | NCT01657331 | 29 |
| BV-ipilimumab | 1 | 21 | 76 | 57 | NCT01896999 | 30 |
| BV-nivolumab | 1 | 18 | 88 | 61 | NCT01896999 | 30 |
| BV-nivolumab-ipilimumab | 1 | 22 | 82 | 73 | NCT01896999 | 30 |
| Anti PD-1 monotherapy and combination therapy in R/R cHL | ||||||
| Nivolumab | 2 | 243 | 69 | 16 | NCT02181738 | 16 |
| Pembrolizumab | 2 | 210 | 69 | 22 | NCT02453594 | 18 |
| Nivolumab+ipilimumab | 1b | 31 | 74 | 23 | NCT01592370 | 31 |
| Pembrolizumab+vorinostat | 1 | 9* | 100 | 44 | NCT03150329 | 32 |
| Camrelizumab+decitabine | 2 | NCT03250962 | 33 | |||
| Anti-PD-1 naive | — | 42 | 95 | 71 | — | |
| Prior anti-PD-1 | — | 25 | 52 | 28 | — |
CRR, complete response rate.
Includes patients with both prior PD-1 blockade and PD-1 refractory.
Case 2
A 30-year-old woman was diagnosed with stage IV cHL and received 2 cycles of ABVD (ABVD×2)/AVD×4, achieving a CMR at EOT. Seven months later, the patient developed fever and night sweats. PET-CT showed hypermetabolic adenopathy above and below the diaphragm and fluorodeoxyglucose uptake in multiple vertebrae. Lymph node biopsy specimens confirmed relapsed cHL.
Novel agent–based salvage therapy for R/R cHL after frontline therapy
There are many salvage therapy options for R/R cHL, with no randomized, controlled trials in the modern era to guide practice. The efficacy of conventional platinum- or gemcitabine-based chemotherapy regimens is similar, with ORR and CR rates by PET ranging between 70% and 89% and 54% to 73%, respectively.34-39 Achievement of a CMR before transplant is a critical prognostic factor in auto-HCT outcome.40-43 Several studies incorporating BV and/or PD-1 blockade into initial salvage therapy have aimed to improve the CMR rate before auto-HCT.
Sequential and combination BV-based salvage regimens have been studied (Table 2).44-52 Although a minority of patients have a CR with BV alone as initial salvage therapy (27% to 43%),44-46 the CMR rates after sequential BV chemotherapy or combined BV/chemotherapy range from 68% to 83%. The primary toxicities observed with these regimens are hematologic.
Novel agent–based salvage regimens in second-line therapy in cHL
| Regimen . | Phase . | N . | Primary refractory, n . | Relapsed, n . | CMR, % . | PFS, % . | Reference . |
|---|---|---|---|---|---|---|---|
| BV based salvage regimens | |||||||
| Sequential BV and chemotherapy | |||||||
| BV→augmented ICE | 2 | 45 | 25 | 20 | 76* | 82 at 3 y | 44 |
| BV→salvage therapy | 2 | 57 | 35 | 22 | 74† | 71 at 2 y | 45 |
| Combination BV and chemotherapy/ novel agents | |||||||
| BV + ICE | |||||||
| BV-ICE x 2 | 1/2 | 16 | 11 | 5 | 69 | Not reported | 47 |
| BV-ICE x 2-3 | 2 | 42‡ | 12 | 30 | 69 | 69 at 1 y | 48 |
| BV + ESHAP | 1/2 | 66 | 40 | 26 | 70 | 71 at 30 mo | 49 |
| BV + DHAP | 2 | 55 | 23 | 32 | 81 | 74 at 2 y | 50 |
| BV + Gemcitabine | 1/2 | 45§ | 29 | 16 | 67 | Not reached | 51 |
| BV + bendamustine | 1/2 | 55 | 28 | 27 | 74 | 62.6 at 2 y | 52 |
| BV + nivolumab | 1/2 | 91 | 38 | 53 | 67 | 77 at 3 y | 53,54 |
| Anti–PD-1 based salvage regimens | |||||||
| N±ICE‖ | 2 | 39 | 18 | 21 | 86¶ | 79 at 1 y | 58 |
| Pem-ICE | 2 | 23 | 8 | 15 | 96 | Not reported | 59 |
| Pem-GVD# | 2 | 39 | 16 | 23 | 95 | Not reached | 60,61 |
| Regimen . | Phase . | N . | Primary refractory, n . | Relapsed, n . | CMR, % . | PFS, % . | Reference . |
|---|---|---|---|---|---|---|---|
| BV based salvage regimens | |||||||
| Sequential BV and chemotherapy | |||||||
| BV→augmented ICE | 2 | 45 | 25 | 20 | 76* | 82 at 3 y | 44 |
| BV→salvage therapy | 2 | 57 | 35 | 22 | 74† | 71 at 2 y | 45 |
| Combination BV and chemotherapy/ novel agents | |||||||
| BV + ICE | |||||||
| BV-ICE x 2 | 1/2 | 16 | 11 | 5 | 69 | Not reported | 47 |
| BV-ICE x 2-3 | 2 | 42‡ | 12 | 30 | 69 | 69 at 1 y | 48 |
| BV + ESHAP | 1/2 | 66 | 40 | 26 | 70 | 71 at 30 mo | 49 |
| BV + DHAP | 2 | 55 | 23 | 32 | 81 | 74 at 2 y | 50 |
| BV + Gemcitabine | 1/2 | 45§ | 29 | 16 | 67 | Not reached | 51 |
| BV + bendamustine | 1/2 | 55 | 28 | 27 | 74 | 62.6 at 2 y | 52 |
| BV + nivolumab | 1/2 | 91 | 38 | 53 | 67 | 77 at 3 y | 53,54 |
| Anti–PD-1 based salvage regimens | |||||||
| N±ICE‖ | 2 | 39 | 18 | 21 | 86¶ | 79 at 1 y | 58 |
| Pem-ICE | 2 | 23 | 8 | 15 | 96 | Not reported | 59 |
| Pem-GVD# | 2 | 39 | 16 | 23 | 95 | Not reached | 60,61 |
CMR was determined by PET scan.
DHAP, dexamethasone, cytarabine, cisplatin; ESHAP, etoposide, cytarabine, cisplatin, methylprednisolone; N±ICE, nivolumab with or without ICE.
27% achieved CMR to BV alone, and 76% to both.
43% achieved CMR to BV alone, and 74% to both.
Only 39 patients were evaluable for efficacy.
Only 42 patients were evaluable for efficacy.
Patients received nivolumab 3 mg/kg every 2 weeks for 6 cycles. Patients in CR at cycle 6 proceeded to auto-HCT; those not in CR received nivo+ICE for 2 cycles.
End of nivolumab alone CR rate, 70%; end of nivo+ICE CR rate, 86%.
Thirty-seven patients were evaluable for response.
BV (1.8 mg/kg) and nivolumab (3 mg/kg), combined as initial salvage therapy every 3 weeks for 4 cycles (n = 91), yielded an ORR of 85% and CR rate of 67%,53 with excellent durability of responses (3-year PFS of 77% in the entire cohort and 61% in the primary refractory subgroup), including a 3-year PFS of 91% among patients who proceeded directly to auto-HCT after BV+nivolumab (BV+nivo). The combination was well tolerated with 14% of patients requiring systemic steroids for irAEs and no treatment discontinuation related to irAEs. Infusion-related reactions were common (44%), but most were grade 1/2.53,54 Preliminary results of BV+nivo as first salvage therapy in children, adolescents, and young adults (www.clinicaltrials.gov, #NCT02927769) followed by BV+bendamustine in patients not in CR demonstrated a promising CR rate of 89% before auto-HCT (n = 44).55
How do we incorporate novel agents into salvage therapy for R/R cHL?
Relapsed, novel agent–naïve cHL
There are no randomized, controlled data to guide decisions about first salvage therapy for patients with R/R cHL. Regimens are selected based on physician or patient preference (eg, desire to avoid chemotherapy or inpatient hospitalization). In case 2, the patient had early relapse of cHL after receiving PET-adapted A(B)VD therapy and had not previously received novel agents. Salvage combination chemotherapy remains the standard approach; however, we would also consider use of BV+nivo based on the high likelihood of achieving CR and excellent PFS, outpatient administration, and its favorable toxicity profile. This use of BV or nivolumab has not been approved but is supported by guidelines.56
Primary refractory, novel agent–naïve cHL
Some salvage regimens are associated with lower CR rates in patients with primary refractory cHL compared with relapsed cHL, including some chemotherapy regimens (BeGeV, 59% refractory vs 84% relapsed),39 BV+chemo regimens (BV+bendamustine, 64% refractory vs 84% relapsed),52 and BV+nivo (48% refractory vs 71% relapsed). Although PFS has not been different between patients with primary refractory cHL and those with relapsed cHL after most salvage therapies (and autologous stem cell transplant), the 21-month PFS in patients with primary refractory cHL after BV+nivo as first salvage therapy was 65% vs 97% in patients with relapsed cHL. At present, chemotherapy-based salvage remains the standard for these patients, although novel salvage approaches remain satisfactory options supported by guidelines.56,57
R/R cHL with prior exposure to novel agents
Some patients will have received novel agents as part of frontline therapy. In these patients, we would use a standard chemotherapy-based salvage regimen at the present time. However, with promising early data on anti-PD-1–based salvage independent of BV,58-61 anti-PD-1–based salvage may be a future option for patients with frontline BV failure. Of note, the duration of salvage therapy with novel salvage regimens is typically longer (eg, 4 cycles over 12 weeks with BV+nivo) compared with conventional chemotherapy (eg, 3 cycles/9 weeks with ICE). Priorities regarding therapy duration and toxicities should be considered when selecting a salvage regimen.
Novel agents as post–auto-HCT consolidation therapy
In the phase 3 double-blind, randomized AETHERA study, patients with high-risk (defined as primary refractory, relapse <12 months after initial therapy, or extranodal relapse) R/R cHL (n = 329, all BV naïve) received BV (1.8 mg/kg every 3 weeks) or placebo for up to 16 cycles as consolidation after auto-HCT.9 The median PFS was 42.9 months after BV, compared with 24.1 months with placebo (HR, 0.57) and sustained PFS benefit was observed with long-term follow-up (5-year PFS 59% vs 41%; HR, 0.52),9,10 resulting in USFDA approval for BV as a post–auto-HCT consolidation therapy. Patients with ≥2 adverse risk factors (eligibility factors, B symptoms at relapse, less than CR at auto-HCT, and ≥2 salvage therapies) benefitted most from BV consolidation (HR, 0.42).9,10 PD-1 blockade has also been evaluated as post–auto-HCT consolidation in patients with R/R cHL, alone or in combination with BV. In separate phase 2 studies, pembrolizumab (8 cycles, n = 30) or BV+nivo (8 cycles; n = 59) resulted in 81% and 92% 19-month PFS, respectively.62,63
How do we incorporate novel agents as post–auto-HCT consolidation therapy for R/R cHL?
BV consolidation after auto-HCT is approved for use in high-risk patients with cHL with primary refractory disease, relapse within 12 months of frontline therapy completion, or extranodal relapse. In case 2, the patient had 3 modified AETHERA high-risk factors (early extranodal relapse with B symptoms). We would recommend BV consolidation for this BV-naïve patient, especially considering the benefit observed in the post hoc analysis of patients with ≥2 modified AETHERA risk factors. AETHERA enrolled only BV-naïve patients, so the benefit of BV consolidation is unclear in patients with prior BV exposure. We do not recommend BV consolidation in patients who have demonstrated BV resistance (eg, best response stable disease/PD or PD <3 months after BV) before auto-HCT. In high-risk patients who remain BV sensitive after a short course of BV before auto-HCT (eg, CR to BV-based salvage), we consider BV consolidation, although there are no data to support its use.64 We recommend counting salvage BV cycles toward the maximum 16 cycles of consolidation (ie, maximum 12 consolidation cycles after 4 cycles of salvage BV). Although results are promising thus far, because limited data are available, we do not currently recommend post–auto-HCT consolidation with PD-1 blockade.
Case 3
A 22-year-old man presented with a 7-week history of pruritus, fatigue, fevers, night sweats, and a neck mass. On examination, he had palpable bilateral cervical and axillary lymphadenopathy. Laboratory values demonstrated: white blood cell count 11 × 103/µL, absolute lymphocyte count 0.4 × 103/µL, hemoglobin 9 g/dL, and albumin 2.8 g/dL. PET-CT demonstrated hypermetabolic cervical, axillary, and mediastinal lymphadenopathy and multiple fluorodeoxyglucose-avid bone lesions. Lymph node biopsy confirmed a diagnosis of nodular sclerosing cHL.
Standard frontline therapy for cHL
Combination chemotherapy, with or without radiotherapy is standard treatment of newly diagnosed cHL. PET-adapted approaches allow for de-escalation of therapy in patients with a negative interim PET and therapy intensification in patients with early evidence of chemoresistance. In early-stage cHL, ABVD with radiotherapy or PET-adapted chemotherapy yields cure rates of 85% to 90%,5,65,66 depending on disease bulk and other risk factors. In patients with advanced-stage cHL, PET-adapted approaches starting with ABVD or escalated-BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone), are associated with 75% to 90% long-term PFS.67-69
BV in frontline treatment of cHL
ECHELON-1 (n = 1334) was a randomized phase 3 trial comparing 6 cycles of BV+AVD to ABVD (non-PET adapted) in newly diagnosed, advanced-stage cHL. The primary end point was 2-year modified PFS (mPFS), with events defined as death, disease progression, or <CR at EOT followed by subsequent antilymphoma therapy. The USFDA approved BV+AVD as frontline treatment of advanced-stage cHL, as it resulted in a 2-year mPFS of 82.1% compared with 77.2% after ABVD. In a post hoc analysis, traditional 3-year PFS was higher after BV+AVD (83% vs 76.1% with ABVD). Notable toxicities with BV+AVD compared with ABVD included more neuropathy (all grades, 67% vs 43%; grade 3, 11% vs 2%), febrile neutropenia (19% vs 8%), and grade ≥3 infection (18% vs 10%), but less pulmonary toxicity (2% vs 7%). Prophylactic granulocyte colony-stimulating factor with BV+AVD reduced the rate of grade ≥3 neutropenia to 29% from 70% and febrile neutropenia to 11% from 21% and is recommended.70 Among patients randomized to the BV+AVD arm who developed peripheral neuropathy, 62% had complete resolution and 17% had improvement without resolution of symptoms at last follow-up. The 5-year data on fertility and secondary malignancies showed no concerning signals,71 but longer follow-up and additional data are needed.72,73 BV has also been studied as part of frontline treatment of early-stage cHL in combination with AVD (n = 34; 3-year PFS, 94%) or as consolidation after ABVD (n = 41; 3-year PFS, 92%) with promising results observed in phase 2 studies.74,75 The randomized phase 3 UK RADAR study is a PET-adapted trial that will determine whether BV+AVD, with or without radiotherapy, improves the PFS and CMR rate compared with ABVD, with or without radiotherapy in early-stage cHL.
Finally, BV has been studied in combination with variations of escalated BEACOPP in frontline therapy of advanced-stage cHL. BreCADD (BV, etoposide, doxorubicin, cyclophosphamide, vincristine, dacarbazine, and dexamethasone) had a more favorable toxicity profile76 and is being evaluated against escalated BEACOPP in a randomized phase 3 HD21 trial (#NCT02661503).
PD-1 blockade in frontline treatment of cHL
Increasing degrees of 9p24.1 aberration are associated with a higher probability of frontline chemotherapy failure in cHL.77 Hence, incorporating PD-1 blockade into frontline therapy may overcome adverse biological features and potentially improve outcomes. In CheckMate 205 (cohort D; n = 51), patients with newly diagnosed stage 2B-4 cHL received nivolumab every 2 weeks for 4 doses followed by nivolumab plus AVD (nivo+AVD) for every 2 weeks for 12 doses. After nivo+AVD combotherapy, the CR rate was 67% by central review and 80% by investigators. The 9-month mPFS was 92%, and subsequent follow-up showed that 21-month investigator-assessed PFS was 83%. Grade ≥3 AEs were uncommon except for neutropenia (49%) and febrile neutropenia (10%); the most common irAEs were hypothyroidism or hyperthyroidism (26%), all grade 1/2.78,79
Sequential pembrolizumab followed by AVD was evaluated in a phase 2 study of newly diagnosed cHL (n = 30; early-stage unfavorable, 12; advanced, 18), with 3 cycles of pembrolizumab every 3 weeks, followed by 4 to 6 cycles of AVD. The CMR was 37% after pembrolizumab monotherapy and 100% after 2 cycles of AVD.80 A randomized phase 2 study evaluated combination (nivo+AVD×4) vs sequential (nivolumab×4, nivo+AVD×2, and AVD×2) nivolumab with AVD in patients with early-stage unfavorable cHL with similar 12-month PFS after concomitant (100%) and sequential (98%) therapy81 (Table 3).
Novel agent–based treatment in newly diagnosed cHL
| Regimen . | Phase . | n . | Stage . | Median age, y . | CRR, % . | PFS, % . | Reference . |
|---|---|---|---|---|---|---|---|
| BV+AVD vs ABVD | 3 | 1334 | III-IV | 35 vs 37 | 73 vs 71 | 83 vs 76.1 at 3 y | 72,73 |
| Nivo→nivo+AVD | 2 | 51 | IIB*-IV | 37 | 67† | 83 at 21 mo | 78,28 |
| Pem→AVD | 2 | 30 | IIB*-IV | 30 | 100‡ | 100 at a median follow-up of 22.5 mo | 80 |
| Nivo+AVD concomitant vs sequential§ | 2 | 110 | I-II (unfavorable) | 26 vs 27 | 83 vs 84 | 100 vs 98 at 12 mo | 81 |
| BV+AVD | 2 | 34 | I-II* (nonbulky) | 36 | 91 | 94 at 3 y | 74 |
| ABVD→BV | 2 | 40 | I-II* (nonbulky) | 29 | 95 | 92 at 3 y | 75 |
| Regimen . | Phase . | n . | Stage . | Median age, y . | CRR, % . | PFS, % . | Reference . |
|---|---|---|---|---|---|---|---|
| BV+AVD vs ABVD | 3 | 1334 | III-IV | 35 vs 37 | 73 vs 71 | 83 vs 76.1 at 3 y | 72,73 |
| Nivo→nivo+AVD | 2 | 51 | IIB*-IV | 37 | 67† | 83 at 21 mo | 78,28 |
| Pem→AVD | 2 | 30 | IIB*-IV | 30 | 100‡ | 100 at a median follow-up of 22.5 mo | 80 |
| Nivo+AVD concomitant vs sequential§ | 2 | 110 | I-II (unfavorable) | 26 vs 27 | 83 vs 84 | 100 vs 98 at 12 mo | 81 |
| BV+AVD | 2 | 34 | I-II* (nonbulky) | 36 | 91 | 94 at 3 y | 74 |
| ABVD→BV | 2 | 40 | I-II* (nonbulky) | 29 | 95 | 92 at 3 y | 75 |
Data represent BV and anti-PD-1 therapies in newly diagnosed cHL.
CRR, complete response rate; DTIC, dacarbazine; Pem, pembrolizumab.
Includes unfavorable cHL.
CR rate 67% by central review and 80% by investigators.
PET-CR rate was 37% after pembrolizumab monotherapy and 100% after AVD × 2 cycles.
Concomitant nivo+AVD received 4 cycles of nivo+AVD; the sequential arm received nivolumab × 4 cycles→nivo+AVD × 2 cycles→AVD × 2 cycles followed by 30 Gy involved-site radiation therapy.
Novel agents in frontline treatment of elderly/frail patients with cHL
Conventional chemotherapy is associated with inferior outcomes in newly diagnosed elderly/frail patients with cHL, because of toxicity and higher rates of relapse.82,83 In the E2496 study comparing Stanford V to ABVD in advanced-stage cHL, patients ≥60 years or age had 9% treatment-related mortality, 48% 5-year failure-free survival, and 58% 5-year OS, compared with 0.3%, 74%, and 90% in younger patients, respectively.83
BV monotherapy, sequential BV and chemotherapy, combination BV+chemotherapy, and BV+nivo have been studied in newly diagnosed elderly (≥60 years)/frail patients, although studies have used variable enrollment criteria (ie, elderly and/or frail and/or ineligible for chemotherapy), affecting interpretation and application of these data (Table 4). BV monotherapy is associated with ORR and CR rates of 84% to 92% and 26% to 73%, respectively, in newly diagnosed elderly/frail cHL patients, with a median PFS of 7.3 to 10.5 months.84,85 The most common toxicity in both studies was neuropathy. BV+dacarbazine (375 mg/m2) and BV+bendamustine (90 or 70 mg/m2) resulted in similar ORR and CR rates (BV+dacarbazine, 100% ORR/62% to 69% CR; BV+bendamustine 100% ORR/88% CR), but BV+bendamustine is associated with increased toxicity in elderly patients with standard BV dosing (1.8 mg/kg dose; 65% serious AEs).86 The durability of responses is improved when single-agent chemotherapy is added to BV, with a median PFS of 46.8 months after BV+dacarbazine and 40.3 months after BV (1.8 mg/kg)+bendamustine, and 2-year PFS of 54% after BV (1.2 mg/kg)+bendamustine.86-88
Treatment in newly diagnosed elderly/frail cHL
| Regimen . | Phase . | n . | Stage . | Median age, y . | CRR, % . | PFS . | Frail . | Reference . |
|---|---|---|---|---|---|---|---|---|
| BV and anti PD-1 therapies in elderly/frail patients | ||||||||
| BV | 2 | 27 | I-IV | 78 | 73 | Median, 10.5 mo | 81% were impaired in ≥1 aspect of GA | 84 |
| BV (BREVITY) | 2 | 38 | II-IV | 76 | 26 | Median, 7.3 mo | Median CIRS-G score was 6 in evaluable patients | 85 |
| BV (1.2 mg/m2) +bendamustine (HALO) | 1/2 | 59 | II-IV | 70 | 63 | 54% at 2 y | 79% | 87 |
| BV+DTIC | 2 | 19 | I-IV | 69 | 68 | Median PFS, 46.8 mo | 50% | 86,88 |
| BV (1.8 mg/m2) +bendamustine (70 mg/m2) | 2 | 20 | I-IV | 75 | 88 | Median PFS, 40.3 mo | 45% | 86,88 |
| BV+nivo (≤16 cycles) | 2 | 21 | II-IV | 72 | 79 | Median PFS not reached | GA not reported* | 88 |
| BV+nivo (8 cycles) | 2 | 46 | II-IV | 71 | 68 | Median PFS, 21.8 mo | GA not assessed† | 89 |
| Sequential BV→AVD‡ | 2 | 48 | II-IV | 69 | 81 | 85% at 2 y | Median CIRS-G score was 7 | 90 |
| Standard chemotherapies in elderly/frail patients | ||||||||
| ABVD×6-8§ | 3§ | 23 | II-IV | 66 | 65 | 64% at 5 y | — | 83 |
| Stanford V§ | 3§ | 21 | II-IV | 64 | 62 | 51% at 5 y | — | 83 |
| ABVD×2+IFRT‖ | 3‖ | 70 | I-II | 64 | 96 | 70% at 5 y | — | 94 |
| AVD×2+IFRT¶ | 3¶ | 82 | I-II | 66 | 98 | 70% at 5 y | — | 94 |
| PVAG×6-8 | 2 | 59 | II-IV | 68 | 78 | 58% at 3y | — | 96 |
| Regimen . | Phase . | n . | Stage . | Median age, y . | CRR, % . | PFS . | Frail . | Reference . |
|---|---|---|---|---|---|---|---|---|
| BV and anti PD-1 therapies in elderly/frail patients | ||||||||
| BV | 2 | 27 | I-IV | 78 | 73 | Median, 10.5 mo | 81% were impaired in ≥1 aspect of GA | 84 |
| BV (BREVITY) | 2 | 38 | II-IV | 76 | 26 | Median, 7.3 mo | Median CIRS-G score was 6 in evaluable patients | 85 |
| BV (1.2 mg/m2) +bendamustine (HALO) | 1/2 | 59 | II-IV | 70 | 63 | 54% at 2 y | 79% | 87 |
| BV+DTIC | 2 | 19 | I-IV | 69 | 68 | Median PFS, 46.8 mo | 50% | 86,88 |
| BV (1.8 mg/m2) +bendamustine (70 mg/m2) | 2 | 20 | I-IV | 75 | 88 | Median PFS, 40.3 mo | 45% | 86,88 |
| BV+nivo (≤16 cycles) | 2 | 21 | II-IV | 72 | 79 | Median PFS not reached | GA not reported* | 88 |
| BV+nivo (8 cycles) | 2 | 46 | II-IV | 71 | 68 | Median PFS, 21.8 mo | GA not assessed† | 89 |
| Sequential BV→AVD‡ | 2 | 48 | II-IV | 69 | 81 | 85% at 2 y | Median CIRS-G score was 7 | 90 |
| Standard chemotherapies in elderly/frail patients | ||||||||
| ABVD×6-8§ | 3§ | 23 | II-IV | 66 | 65 | 64% at 5 y | — | 83 |
| Stanford V§ | 3§ | 21 | II-IV | 64 | 62 | 51% at 5 y | — | 83 |
| ABVD×2+IFRT‖ | 3‖ | 70 | I-II | 64 | 96 | 70% at 5 y | — | 94 |
| AVD×2+IFRT¶ | 3¶ | 82 | I-II | 66 | 98 | 70% at 5 y | — | 94 |
| PVAG×6-8 | 2 | 59 | II-IV | 68 | 78 | 58% at 3y | — | 96 |
CIRS-G, Cumulative Illness Rating Scale-Geriatric comorbidity score; DTIC, dacarbazine; GA, geriatric assessment; IFRT, involved field radiation therapy.
Patients ≥60 year of age who were considered unfit for conventional chemotherapy were included in the study.
Patients were selected based on abnormal organ function that would render them poor candidates for standard chemotherapy.
Patients received 2 cycles of BV followed by 6 cycles of AVD and then 4 cycles of BV consolidation in those who responded.
Secondary analysis of E2496 data.
Secondary analysis of GHSG HD10 study data.
Secondary analysis of GHSG HD13 study data.
Frontline BV+nivo has been evaluated in 2 phase 2 studies and is associated with best ORR/CR rates of 91% to 95% and 65% to 79%, respectively. More grade ≥3 AEs were observed in elderly patients, most commonly elevated lipase (19%), neutropenia (17%), and peripheral neuropathy (11% to 14%; 48% all grades).88 In a study of fixed-duration BV+nivo (8 cycles, n = 46), the median PFS was 18.3 months, but the study did not meet its prespecified goal of 65% ORR at EOT, with only 61% and 48% of patients remaining in response and CR, respectively.89
Finally, a phase 2 study evaluated 2 cycles of BV followed by 6 cycles of AVD and then 4 cycles of BV consolidation in elderly patients with newly diagnosed cHL (n = 48). The ORR/CR rate after BV×2/AVD×6 were 95% and 90% with a 2-year PFS and OS of 84% and 93%, respectively; 77% of patients completed AVD×6, 73% received ≥1 dose of BV consolidation, and 42% experienced a serious AE (most commonly neutropenia or infection).90
How do we incorporate novel agents for patients with newly diagnosed cHL?
Early-stage cHL
In young, fit patients with early-stage cHL, we do not recommend incorporation of BV or PD-1 blockade because of the dearth of randomized, controlled data with sufficient long-term follow-up at this time.
Advanced-stage cHL
In patients with newly diagnosed advanced-stage cHL, treatment selection requires consideration of the risks and benefits of the various options available in discussion with the patient. ABVD has been the standard in North America and bleomycin can be safely discontinued after 2 cycles in patients with a negative interim PET scan.4 However, patients with a positive interim PET (PET2+) scan have dismal outcomes with ABVD continuation,66 and the alternative is escalation to BEACOPP, a regimen associated with increased toxicity compared with ABVD. Although BV+AVD was compared with ABVD without PET adaptation and the primary end point of the ECHELON-1 study was the unfamiliar mPFS, both the mPFS and traditionally defined PFS were higher than those in the control arm.91 Without escalation to BEACOPP, PET2+ patients had a 3-year PFS of 67.7% in ECHELON-1 (58% 2-year mPFS)73 compared with PET2+ patients with 5-year PFS of 70.7% and 66% in the AHL2011 and S0816 studies, respectively, and a 67.5% 3-year PFS in RATHL.4,73,92 Major drawbacks of BV+AVD include increased toxicity compared with ABVD (ie, peripheral neuropathy, neutropenia, and infections), the requirement for granulocyte colony-stimulating factor use, and high cost.
In case 3, the patient had stage IV cHL with several high-risk features and an International Prognostic Score of 6. PET-adapted ABVD (eg, RATHL)- or BEACOPP-based approaches (eg, AHL2011) remain standard options for this patient. However, in secondary analyses of the ECHELON-1 study, younger patients with higher risk disease appeared to benefit the most from BV+AVD. Therefore, the high-risk patient described in case 3 is the type of patient in whom we strongly consider use of BV+AVD. As just stated, we do not recommend the use of PD-1 blockade as part of initial frontline therapy for young, fit patients with advanced-stage cHL based on lack of supporting data. The phase 3 randomized S1826 study is evaluating BV+AVD vs nivo+AVD as frontline treatment for advanced-stage cHL (#NCT03907488).
Elderly cHL
In elderly or frail patients or those with comorbidities, we base decision making on whether the patient can tolerate combination chemotherapy. If yes, for early-stage favorable elderly/unfit patients, 2 cycles of ABVD followed by radiation therapy93 is relatively well tolerated and associated with excellent outcomes. For patients with nonbulky, early-stage disease, PET-adapted ABVD (3-4 cycles as per RAPID or CALGB 50604)5,66 can be considered, but 4 cycles of bleomycin is associated with increased toxicity and the risks vs benefits of using bleomycin in elderly patients or in the presence of comorbidities must be weighed.94 For advanced-stage patients or those who can tolerate chemotherapy but not bleomycin, we use the sequential BV-AVD regimen,90 because of its curative potential as a full systemic course of therapy compared with BV monotherapy or doublets. If the patient cannot tolerate combination chemotherapy, we recommend BV-based therapy, preferably BV+dacarbazine, because it is associated with a longer duration of response or BV monotherapy for very elderly/unfit patients. If the patient’s performance status improves after treatment with BV monotherapy, it is reasonable to transition to sequential BV-AVD.
Conclusions
The scenarios described herein represent our management recommendations for common questions that currently arise regarding how to integrate novel therapies into the treatment of cHL. Dilemmas remain because the field is evolving so rapidly that a patient with cHL encountered in the clinic may have multiply R/R cHL with no prior exposure to novel agents or may have received a novel agent as frontline or salvage treatment, which prevents uniform recommendations for all patients at a particular time point. There is also a lack of randomized controlled data to guide decisions, although studies such as KEYNOTE-204 are aiming to bridge the gap. A critical question for the future of cHL management is the optimal timing for the use of BV and PD-1 blockade and what the best combination partners are. As we treat more patients with these drugs earlier in the disease course, patients with R/R cHL will increasingly have BV- or anti-PD-1–resistant cHL and approaches to overcoming resistance will be necessary. As we better understand the biology of the novel agents, we may identify targetable mechanisms of resistance, and some BV- and anti-PD-1-based combinations have demonstrated early promise.32,33,95 We must also accept that many patients have cHL that is not cured by the current novel agents and that newer targeted therapies for cHL are needed.
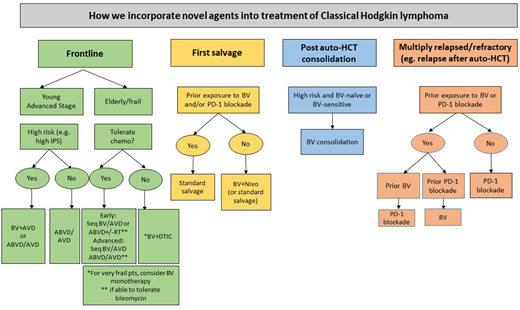
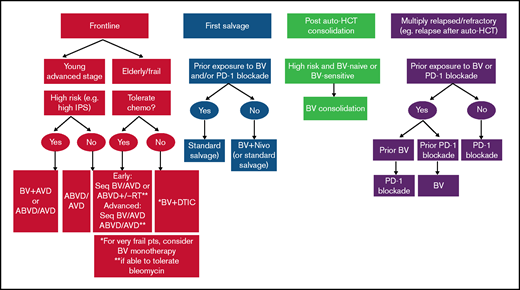
As we await data to clarify the optimal role and timing of BV and PD-1 blockade in cHL, a proposed algorithm for how to incorporate novel agents into the management of cHL is shown in Figure 1.
How we incorporate novel agents into treatment of classical Hodgkin lymphoma.
Acknowledgments
The authors thank Philippe Armand for his thoughtful review of the manuscript.
A.F.H. is supported by the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award.
Authorship
Contribution: N.E. and A.F.H. wrote and edited the manuscript.
Conflict-of-interest disclosure: A.F.H. has served in a consulting or advisory role for Bristol-Myers Squibb, Merck, Seattle Genetics, Karyopharm, and Gilead/Kite Pharma and has received a portion of the institutional research funding provided by Bristol-Myers Squibb, Genentech/Roche, Merck, Seattle Genetics, Gilead/Kite Pharma, and ADC Therapeutics.
Correspondence: Alex F. Herrera, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: aherrera@coh.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal