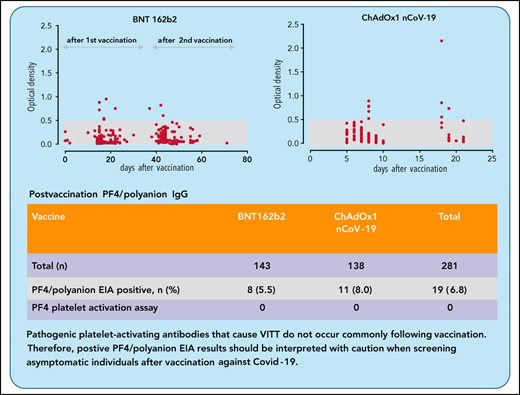
Key Points
Low-titer PF4/polyanion antibodies occur after vaccination with ChAdOx1 nCoV-19 and BNT162b2.
These PF4/polyanion antibodies do not activate platelets and may have little relevance for the diagnosis of VITT.
Vaccination using the adenoviral vector COVID-19 vaccine ChAdOx1 nCoV-19 (AstraZeneca) has been associated with rare vaccine-induced immune thrombotic thrombocytopenia (VITT). Affected patients test strongly positive in platelet factor 4 (PF4)/polyanion enzyme immunoassays (EIAs), and serum-induced platelet activation is maximal in the presence of PF4. We determined the frequency of anti-PF4/polyanion antibodies in healthy vaccinees and assessed whether PF4/polyanion EIA+ sera exhibit platelet-activating properties after vaccination with ChAdOx1 nCoV-19 (n = 138) or BNT162b2 (BioNTech/Pfizer; n = 143). In total, 19 of 281 participants tested positive for anti-PF4/polyanion antibodies postvaccination (All: 6.8% [95% confidence interval (CI), 4.4-10.3]; BNT162b2: 5.6% [95% CI, 2.9-10.7]; ChAdOx1 nCoV-19: 8.0% [95% CI, 4.5% to 13.7%]). Optical densities were mostly low (between 0.5 and 1.0 units; reference range, <0.50), and none of the PF4/polyanion EIA+ samples induced platelet activation in the presence of PF4. We conclude that positive PF4/polyanion EIAs can occur after severe acute respiratory syndrome coronavirus 2 vaccination with both messenger RNA- and adenoviral vector-based vaccines, but many of these antibodies likely have minor (if any) clinical relevance. Accordingly, low-titer positive PF4/polyanion EIA results should be interpreted with caution when screening asymptomatic individuals after vaccination against COVID-19. Pathogenic platelet-activating antibodies that cause VITT do not occur commonly following vaccination.
Introduction
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are a cornerstone in controlling the SARS-CoV-2 pandemic.1-4 By March 2021, the European Medical Agency approved 4 vaccines to prevent symptomatic COVID-19, all encoding the spike protein antigen of SARS-CoV-2: 2 messenger RNA (mRNA)-based vaccines, BNT162b2 (BioNTech/Pfizer) and mRNA1273 (Moderna), and 2 recombinant vector-based vaccines, the adenovirus type 26 vector Janssen COVID-19 vaccine (Johnson & Johnson) and the recombinant chimpanzee adenoviral vector vaccine ChAdOx1 nCoV-19 (AstraZeneca).
We and others have recently described vaccine-induced immune thrombotic thrombocytopenia (VITT) associated with ChAdOx1 nCoV-19 vaccination. VITT presents between 5 and 20 days following vaccination with thrombocytopenia (median platelet count, ∼20 × 109/L); unusual and severe thromboembolic events, such as cerebral venous sinus thrombosis and splanchnic vein thrombosis; often signs of disseminated intravascular coagulation; and the presence of immunoglobulin G (IgG) antibodies that react strongly in platelet factor 4 (PF4)/polyanion enzyme immunoassays (EIAs) and activate platelets in the presence of PF4.5-7 Hence, VITT shares features with autoimmune heparin-induced thrombocytopenia, including severe thrombocytopenia, disseminated intravascular coagulation, and heparin-independent platelet-activating properties without previous heparin exposure.8 VITT has to date only been described as a rare complication after vaccination with the adenoviral vector-based vaccines ChAdOx1 nCoV-19 and COVID-19 Vaccine Janssen.9
Antibodies of VITT patients bind to PF4 alone, but also to PF4 in PF4/heparin complexes. If VITT is suspected, a screening test for PF4/polyanion antibodies is recommended5,10 to confirm the presence of high-titer anti-PF4 antibodies. It is well known from heparin-induced thrombocytopenia that anti-PF4/polyanion antibodies among heparin-exposed patients are heterogeneous, with only a minority of IgG exhibiting strong heparin-dependent platelet-activating properties. Furthermore, preexisting B cells exist that can produce anti-PF4 antibodies. These B cells are present even in cord blood.11 However, activation of these B cells requires an appropriate antigen and additional cosignals. The frequency of these antibodies is especially high in patients after major surgery, indicating that tissue trauma and inflammation12 provide an important cosignal for induction of anti-PF4 antibody production. After vaccination against COVID-19, inflammatory responses, including fever, chills, and headaches, are frequently reported. This raises the question how frequently platelet-activating anti-PF4/polyanion IgG occurs after vaccination against COVID-19 and whether there is a difference between vector-based and mRNA-based vaccines.
Study design
Participants and procedures
Vaccination of health care workers was performed between January and March 2021 in an institutional program of the University Medicine of Greifswald (UMG). Subjects received either 2 doses of BNT162b2 (Comirnaty, BioNTech/Pfizer) with an interval of 21 to 28 days between doses, or 1 dose of ChAdOx1 nCoV-19 (Vaxzevria, AstraZeneca AB).
This study was performed as a substudy of 2 ongoing clinical studies: the Screening for COVID-19 and Monitoring of Serological Responses to SARS-CoV-2 in Healthcare Workers study (SeCo; #NCT 04370119) and the Adaptive Immune Response against Corona Virus Vaccination study (AICOVI; #NCT04826770). Both studies were conducted at the UMG and assessed the incidence of seroconversion against SARS-CoV-2 among health care workers during the pandemic and/or due to vaccination. Participants gave written and informed consent; the local Ethics Committee approved both studies (BB 068/20 and BB 001/21).
Blood samples from recipients of BNT162b2 were analyzed after the first and the second vaccine dose (SeCo: variable time points; AICOVI: day 0 [before vaccination], 7 and 14 days after 2 doses 28 days apart). Samples from ChAdOx1 nCoV-19 recipients were analyzed before and after the first vaccine dose (SeCo: variable time points; AICOVI: day 0 [before vaccination] and day 7). From a subset of SeCo participants, a prevaccination sample was available. A history of SARS-CoV-2 infection was assessed by questionnaire in both studies. In SeCo, a nasopharyngeal swab for SARS-CoV-2 polymerase chain reaction was obtained at study entry. For each participant, date of vaccination, type of vaccine, date of blood sampling, age, and sex were analyzed. Samples were tested for anti-PF4/polyanion antibodies. In the case of a positive test result, samples were tested for heparin and PF4-dependent platelet-activating antibodies.
Anti-PF4/polyanion antibody testing and platelet activation assay
An in-house IgG-specific PF4/polyanion EIA13 was used to screen for antibodies recognizing PF4 and PF4/heparin complexes.14 Positive results were given in optical density (OD) units, as follows (reference range <0.50): weak reaction, 0.5 to ≤1.0 units; strong reaction >1.0 units). Samples testing positive by PF4/heparin EIA were assessed for platelet-activating antibodies by a washed platelet activation assay in the presence of PF4 (10 µg/mL).5
Results and discussion
In total, 281 vaccinees were assessed, of which 143 (50.9%) received BNT162b2, and 138 (49.1%) received the ChAdOx1 nCoV-19 vaccine; 73.3% were female. Platelet counts were not followed in this study; however, none of the 281 study participants developed thrombosis or clinically evident VITT.
After vaccination, sera of 19 subjects tested positive for anti-PF4/polyanion antibodies corresponding to an overall frequency of 6.8% (95% confidence interval [CI], 4.4-10.3). After BNT162b2 vaccination, sera of 8 of 143 participants tested positive (5.6%; 95% CI 2.9-10.7); and after ChAdOx1 nCoV-19 vaccine, 11 of 138 (8.0%; 95% CI, 4.5% to 13.7%) tested positive (P > .05). Eighteen of 19 antibody-positive vaccinees showed ODs between 0.5 and 1.0 units (weak reaction); however, 1 sample (recipient of ChAdOx1 nCov-19 vaccine) showed an OD >2.0 (Figure 1).
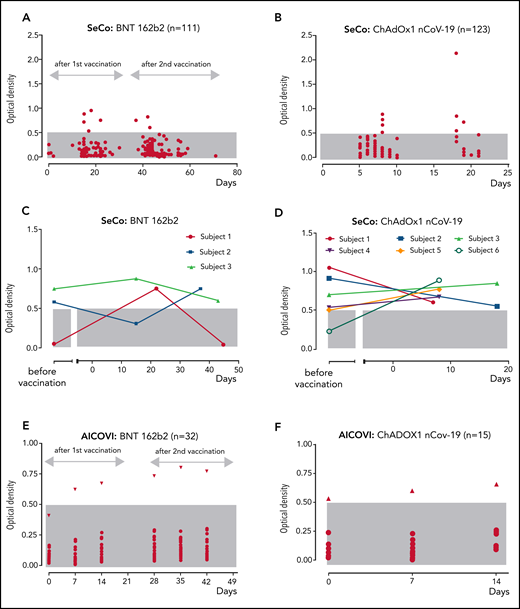
Anti-PF4/polyanion IgG in relation to the time point of vaccination (day 0) with BNT162b2 and ChAdOx1 nCoV-19. An OD of >0.5 units was considered positive (gray shaded area: reference range OD <0.5). SeCo substudy: (A-B) Sera of 111 individuals who were vaccinated twice with BNT162b2 (A); 123 individuals vaccinated once with ChAdOx1 nCoV-19 (B). (C-D) Seropositive subjects with available baseline samples vaccinated with BNT162b2 (C) and vaccinated with ChAdOx1 nCoV-19 (D). Baseline samples were taken at a median of 261 days before BNT162b2 and at a median of 169 days before ChAdOx1 nCoV-19 vaccination. (E-F) AICOVI substudy: Sera of 47 participants tested at prevaccination baseline (day 0) and at days 7 and 14 after 2 doses of BNT162b2 (first dose, day 0; second dose, day 28) (E), and 1 dose of ChAdOx1 nCoV-19 (day 0) (F). Subjects with increasing ODs after vaccination are shown by triangles.
Anti-PF4/polyanion IgG in relation to the time point of vaccination (day 0) with BNT162b2 and ChAdOx1 nCoV-19. An OD of >0.5 units was considered positive (gray shaded area: reference range OD <0.5). SeCo substudy: (A-B) Sera of 111 individuals who were vaccinated twice with BNT162b2 (A); 123 individuals vaccinated once with ChAdOx1 nCoV-19 (B). (C-D) Seropositive subjects with available baseline samples vaccinated with BNT162b2 (C) and vaccinated with ChAdOx1 nCoV-19 (D). Baseline samples were taken at a median of 261 days before BNT162b2 and at a median of 169 days before ChAdOx1 nCoV-19 vaccination. (E-F) AICOVI substudy: Sera of 47 participants tested at prevaccination baseline (day 0) and at days 7 and 14 after 2 doses of BNT162b2 (first dose, day 0; second dose, day 28) (E), and 1 dose of ChAdOx1 nCoV-19 (day 0) (F). Subjects with increasing ODs after vaccination are shown by triangles.
Frequency of anti-PF4/polyanion IgG in vaccinated individuals
| Vaccine . | BNT162b2 . | ChAdOx1 nCoV-19 . | Total . |
|---|---|---|---|
| Total (%) | 143 (50.9) | 138 (49.1) | 281 (100) |
| Age, y (median) | 43 | 45 | 44 |
| Female (%) | 107 (74.8) | 99 (71.7) | 206 (73.3) |
| Postvaccination PF4/polyanion EIA+ (%) | 8 (5.5) | 11 (8.0) | 19 (6.8) |
| SeCo substudy (n, %) | 111 (47.4) | 123 (52.6) | 234 (100) |
| Postvaccination PF4/polyanion EIA+ (%) | 7* (6.3) | 10* (8.1) | 17* (7.3) |
| Baseline samples available | 3 | 6 | 9 |
| Baseline PF4/polyanion EIA+ (%)† | 2 | 4 | 6 |
| AICOVI substudy (n, %) | 32 (68.1) | 15 (31.9) | 47 (100) |
| Postvaccination PF4/polyanion EIA+ (%) | 1 (3.1) | 1 (6.7) | 2 (4.3) |
| Baseline PF4/polyanion EIA+ (%) | 0 (0) | 1 (6.7) | 1 (2.1) |
| Vaccine . | BNT162b2 . | ChAdOx1 nCoV-19 . | Total . |
|---|---|---|---|
| Total (%) | 143 (50.9) | 138 (49.1) | 281 (100) |
| Age, y (median) | 43 | 45 | 44 |
| Female (%) | 107 (74.8) | 99 (71.7) | 206 (73.3) |
| Postvaccination PF4/polyanion EIA+ (%) | 8 (5.5) | 11 (8.0) | 19 (6.8) |
| SeCo substudy (n, %) | 111 (47.4) | 123 (52.6) | 234 (100) |
| Postvaccination PF4/polyanion EIA+ (%) | 7* (6.3) | 10* (8.1) | 17* (7.3) |
| Baseline samples available | 3 | 6 | 9 |
| Baseline PF4/polyanion EIA+ (%)† | 2 | 4 | 6 |
| AICOVI substudy (n, %) | 32 (68.1) | 15 (31.9) | 47 (100) |
| Postvaccination PF4/polyanion EIA+ (%) | 1 (3.1) | 1 (6.7) | 2 (4.3) |
| Baseline PF4/polyanion EIA+ (%) | 0 (0) | 1 (6.7) | 1 (2.1) |
Eight participants who tested positive after vaccination had no available baseline sample (4 with BNT162b2 and 4 with ChAdOx1 nCoV-19).
Only participants with positive PF4/polyanion EIA after vaccination who had an available baseline sample before vaccination were tested.
For 11 of 19 anti-PF4/polyanion IgG+ individuals, prevaccination samples were available (4 received BNT162b, 7 received ChAdOx1 nCoV-19). Here, 7 of 11 sera tested already positive before vaccination. However, 4 individuals showed “seroconversion,” as they tested negative before vaccination and positive after vaccination (ODs before/after: ChAdOx1 nCoV-19: 0.22/0.89 and 0.50/0.77; BNT162b2 0.05/0.75 and 0.41/0.80; Figure 1C-F).
Importantly, none of the sera testing EIA+ for anti-PF4/polyanion antibodies could reproducibly activate platelets in a platelet activation assay in the presence of added PF4. Two sera tested initially weakly/borderline positive, but negative on repeat testing. Of note, platelet-activating antibodies could not be excluded in the 2 positive participants of the AICOVI study, because samples were collected in EDTA. However, ODs were low (OD: 0.7-0.8).
The frequency of positive anti-PF4/polyanion IgG tests in our study appears higher than observed in healthy blood donors. Hursting et al found a frequency of 6.6% seropositive anti-PF4/polyanion samples among 3.795 blood donors,15 but used a test detecting IgG, IgA, and IgM. Our group found no PF4/polyanion IgG+ individuals among 923 blood donors.16 However, blood donors are a preselected group less likely to have underlying inflammatory conditions. In contrast, patients with strong inflammation have a higher likelihood for testing positive for anti-PF4/polyanion IgG. Among intensive care unit patients, 6.3% tested positive at admission and 17.2% tested positive after 10 days of intensive care.17 In addition, the rate of PF4/polyanion IgG seroconversion increases with the severity of trauma.12 Our data suggest that vaccination against COVID-19 leads to anti-PF4 seroconversion in a few subjects and that this may occur after vaccination with either ChAdOx1 nCoV-19 or BNT162b2 as part of the inflammatory response.
A positive anti-PF4/polyanion EIA alone is not sufficient to diagnose VITT, especially if the reactivity strength is low. In contrast to the sera from healthy vaccinees analyzed here, sera from patients with clinically overt VITT are strongly positive by anti-PF4/polyanion EIA (typically ODs >2) and cause strong platelet activation in a washed platelet activation assay in the presence of PF4.5,6 However, high-titer, platelet-activating anti-PF4 antibodies, as observed in VITT, appear to be uncommon in vaccinees (<0.5%). Therefore, PF4/polyanion EIAs results need to be judged in the clinical context, particularly with occurrence of thrombocytopenia and/or thrombosis in a typical time window of 5 to 20 days following vaccination.10 A positive PF4/polyanion EIA result should be interpreted with caution in clinically asymptomatic individuals recently vaccinated against SARS-CoV-2.
Acknowledgments
The authors acknowledge Katja Schulz for her commitment and contribution as study nurse in the SeCo study and are grateful to the technicians of the diagnostics team of the Friedrich Loeffler Institute of Medical Microbiology. The authors thank all study participants for their support.
Members of the SeCo study team include the following individuals: E. Baufeld, M. Beer, J. Bohnert, N. Endlich, M. Groschup, E. A. Idelevich, M. Lerch, T. C. Mettenleiter, M. Nauck, C. Rutscher, W. Schlumberger, M. Tzvetkov, H. Völzke, K. Zimmermann, and M. Zygmunt. Members of the AICOVI Study Team include the following individuals: Kilian A. Wietschel, Kevin Reppschläger, Elmer Antileo, Chiara A. Drechsler, and Erika Friebe. Members of the vaccination team include the following individuals: Ali Aghdassi, Marèn Fricke, Holger Lode, Astrid Radau, Christine Rutscher, and Anna Seidlein. Members of the transfusion medicine platelet laboratory and blood service include the following individuals: Ulrike Strobel, Carmen Freyer, Ricarda Raschke, Ines Warnig, Jessica Fuhrmann, Katrin Stein, and Kathrin Kunze.
This study has been funded by COVIDPROTECT, Ministerium für Wirtschaft, Arbeit, und Gesundheit of the Federal State Mecklenburg–Vorpommern and the Deutsche Forschungsgemeinschaft (German Research Foundation) Projektnummer 374031971-TRR 240.
Authorship
Contribution: T.T., T.E.W., and A.G. designed the study; T.T., L.U., S.H., K.A., and L.S. performed the study; K.B. and N.-O.H. are principal investigators who conducted SeCo; B.M.B. is principal investigator of AICOVI; S.O.K. and C.S. are coordinators of the UMG vaccination program; T.T., L.S., T.E.W., K.A., K.S., and A.G. analyzed the data; T.T., T.E.W., K.S., and A.G. wrote the manuscript; and all authors read and approved the final version of this manuscript.
Conflict-of-interest disclosure: A.G. reports grants from Deutsche Forschungsgemeinschaft and nonfinancial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb, Paringenix, Bayer Healthcare, Gore Inc, Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, Bristol Myers Squibb, Chromatec, and Instrumentation Laboratory, nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH eV outside the submitted work. T.T. reports personal fees and other from Bristol Myers Squibb, personal fees and other from Pfizer, personal fees from Bayer, personal fees and other from Chugai Pharma, other from Novo Nordisk, personal fees from Novartis, other from Daichii Sankyo, all of which are outside the submitted work. T.E.W. reports personal fees from Aspen Canada, Aspen Global, Ergomed, Instrumentation Laboratory, and Octapharma, all of which are outside of the submitted work. K.S. received personal fees from Aspen Germany and travel support from Sobi, outside the submitted work. B.M.B. reports personal fees from Pfizer, Novartis, and GSK outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Thomas Thiele, Institut für Immunologie und Transfusionsmedizin, Abteilung Transfusionsmedizin, Sauerbruchstrasse, 17487 Greifswald, Germany; e-mail: thomas.thiele@med.uni-greifswald.de.
Data are available via direct contact by e-mails to the corresponding author.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
T.T., L.U., and S.H. contributed equally to this study.